Characterization of the intestinal fungal microbiome in patients with hepatocellular carcinoma
- PMID: 36793057
- PMCID: PMC9933289
- DOI: 10.1186/s12967-023-03940-y
Characterization of the intestinal fungal microbiome in patients with hepatocellular carcinoma
Abstract
Objective: Gut mycobiota plays a crucial role in benign liver diseases; however, its correlation with hepatocellular carcinoma (HCC) remains elusive. This study aimed to elucidate fungal differences in patients with HCC-associated cirrhosis compared to cirrhotic patients without HCC and healthy controls.
Methods: The 72 fecal samples from 34 HCC patients, 20 cirrhotic patients, and 18 healthy controls were collected and analyzed using ITS2 rDNA sequencing.
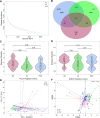
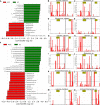
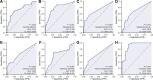
Results: Our results revealed the presence of intestinal fungal dysbiosis with significant enrichment of opportunistic pathogenic fungi such as Malassezia, Malassezia sp., Candida, and C. albicans in HCC patients compared with healthy controls and cirrhosis patients. Alpha-diversity analysis demonstrated that patients with HCC and cirrhosis showed decreased fungal diversity compared to healthy controls. Beta diversity analysis indicated that the three groups exhibited significant segregated clustering. Besides, C. albicans was found to be significantly more abundant in the HCC patients with TNM stage III-IV than those with stage I-II, in contrast to the commensal organism S. cerevisiae. We also confirmed that the HCC patients were successfully classified with an area under the curve value of 0.906 based on the fecal fungal signature. Finally, our animal experiments confirm that aberrant colonization of the intestine by C. albicans and M. furfur can promote the development of HCC.
Conclusions: This study indicates that dysbiosis of the gut mycobiome might be involved in HCC development.
Trial registration: ChiCTR, ChiCTR2100054537. Registered 19 December 2021, http://www.chictr.org.cn/edit.aspx?pid=144550&htm=4.
Keywords: Candida albicans; Gut mycobiome; Hepatocellular carcinoma; ITS2 rDNA sequencing; Liver cirrhosis; Malassezia.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Alterations of gut mycobiota profiles in intrahepatic cholangiocarcinoma.Front Microbiol. 2023 Jan 6;13:1090392. doi: 10.3389/fmicb.2022.1090392. eCollection 2022. Front Microbiol. 2023. PMID: 36687597 Free PMC article.
-
The gut mycobiome of the Human Microbiome Project healthy cohort.Microbiome. 2017 Nov 25;5(1):153. doi: 10.1186/s40168-017-0373-4. Microbiome. 2017. PMID: 29178920 Free PMC article.
-
Gut mycobiome dysbiosis and its impact on intestinal permeability in attention-deficit/hyperactivity disorder.J Child Psychol Psychiatry. 2023 Sep;64(9):1280-1291. doi: 10.1111/jcpp.13779. Epub 2023 Apr 5. J Child Psychol Psychiatry. 2023. PMID: 37016804
-
Gut mycobiota changes in liver diseases: A systematic review.Med Mycol. 2023 Aug 2;61(8):myad071. doi: 10.1093/mmy/myad071. Med Mycol. 2023. PMID: 37463798
-
Fungal infections and the fungal microbiome in hepatobiliary disorders.J Hepatol. 2023 Apr;78(4):836-851. doi: 10.1016/j.jhep.2022.12.006. Epub 2022 Dec 22. J Hepatol. 2023. PMID: 36565724 Free PMC article. Review.
Cited by
-
KHDRBS1 as a novel prognostic signaling biomarker influencing hepatocellular carcinoma cell proliferation, migration, immune microenvironment, and drug sensitivity.Front Immunol. 2024 Apr 10;15:1393801. doi: 10.3389/fimmu.2024.1393801. eCollection 2024. Front Immunol. 2024. PMID: 38660302 Free PMC article.
-
Global research trends and hotspots on human intestinal fungi and health: a bibliometric visualization study.Front Cell Infect Microbiol. 2024 Oct 17;14:1460570. doi: 10.3389/fcimb.2024.1460570. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39483119 Free PMC article.
-
Fungi and tumors: The role of fungi in tumorigenesis (Review).Int J Oncol. 2024 May;64(5):52. doi: 10.3892/ijo.2024.5640. Epub 2024 Mar 29. Int J Oncol. 2024. PMID: 38551162 Free PMC article. Review.
-
"Nutrient-fungi-host" tripartite interaction in cancer progression.Imeta. 2024 Jan 26;3(2):e170. doi: 10.1002/imt2.170. eCollection 2024 Apr. Imeta. 2024. PMID: 38882486 Free PMC article. Review.
-
The predictive value of the neutrophil/eosinophil ratio in cancer patients undergoing immune checkpoint inhibition: a meta-analysis and a validation cohort in hepatocellular carcinoma.Front Immunol. 2025 Jul 21;16:1633034. doi: 10.3389/fimmu.2025.1633034. eCollection 2025. Front Immunol. 2025. PMID: 40761780 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

