Niclosamide from an anthelmintic drug to a promising adjuvant therapy for diabetic kidney disease: randomized clinical trial
- PMID: 36793092
- PMCID: PMC9933377
- DOI: 10.1186/s13098-023-00995-1
Niclosamide from an anthelmintic drug to a promising adjuvant therapy for diabetic kidney disease: randomized clinical trial
Abstract
Background: Diabetic kidney disease (DKD) is a serious complication that begins with albuminuria and often leads to a rapid progressive decline in renal function. Niclosamide is a potent inhibitor of the Wnt/β-catenin pathway, which controls the expression of multiple genes of the renin-angiotensin-aldosterone system (RAAS), which in turn is influences the progression of DKD. This study was conducted to evaluate the effect of niclosamide as adjuvant therapy on DKD.
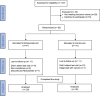
Methods: Out of 127 patients screened for eligibility, 60 patients completed the study. After randomization, 30 patients in the niclosamide arm received ramipril plus niclosamide, and 30 patients in the control arm received ramipril only for 6 months. The primary outcomes were the changes in urinary albumin to creatinine ratio (UACR), serum creatinine, and estimated glomerular filtration rate (eGFR). The secondary outcomes were measurements of urinary matrix metalloproteinase-7 (MMP-7), 8-hydroxy-2'-deoxyguanosine (8-OHdG), and podocalyxin (PCX). Comparisons between the two arms were done using student t-test. Correlation analysis was done using Pearson correlation.
Results: Niclosamide decreased UACR by 24% (95% CI - 30 to - 18.3%) while there was a rise in UACR in the control arm by 11% (95% CI 4 to 18.2%) after 6 months (P < 0.001). Moreover, a significant reduction in MMP-7 and PCX was noticed in the niclosamide arm. Regression analysis revealed a strong association between MMP-7, which is a noninvasive biomarker predicting the activity of the Wnt/β-catenin signaling, and UACR. A 1 mg/dL decline in MMP-7 level was associated with a 25 mg/g lowering in UACR (B = 24.95, P < 0.001).
Conclusion: The addition of niclosamide to patients with diabetic kidney disease receiving an angiotensin-converting enzyme inhibitor significantly reduces albumin excretion. Further larger-scale trials are needed to confirm our results.
Trial registration: The study was prospectively registered on clinicaltrial.gov on March 23, 2020, with identification code NCT04317430.
Keywords: Albuminuria; Diabetic kidney disease; Niclosamide; Wnt/β-catenin.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Nakhoul R, Nakhoul F, Nakhoul N. Diabetic nephropathy from RAAS to autophagy: the era for new players. J Clin Exp Nephrol. 2017;2:43–50. doi: 10.21767/2472-5056.100043. - DOI
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous