Localized strain characterization of cardiomyopathy in Duchenne muscular dystrophy using novel 4D kinematic analysis of cine cardiovascular magnetic resonance
- PMID: 36793101
- PMCID: PMC9933368
- DOI: 10.1186/s12968-023-00922-3
Localized strain characterization of cardiomyopathy in Duchenne muscular dystrophy using novel 4D kinematic analysis of cine cardiovascular magnetic resonance
Abstract
Background: Cardiomyopathy (CMP) is the most common cause of mortality in Duchenne muscular dystrophy (DMD), though the age of onset and clinical progression vary. We applied a novel 4D (3D + time) strain analysis method using cine cardiovascular magnetic resonance (CMR) imaging data to determine if localized strain metrics derived from 4D image analysis would be sensitive and specific for characterizing DMD CMP.
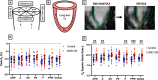
Methods: We analyzed short-axis cine CMR image stacks from 43 DMD patients (median age: 12.23 yrs [10.6-16.5]; [interquartile range]) and 25 male healthy controls (median age: 16.2 yrs [13.3-20.7]). A subset of 25 male DMD patients age-matched to the controls (median age: 15.7 yrs [14.0-17.8]) was used for comparative metrics. CMR images were compiled into 4D sequences for feature-tracking strain analysis using custom-built software. Unpaired t-test and receiver operator characteristic area under the curve (AUC) analysis were used to determine statistical significance. Spearman's rho was used to determine correlation.
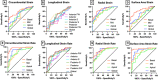
Results: DMD patients had a range of CMP severity: 15 (35% of total) had left ventricular ejection fraction (LVEF) > 55% with no findings of myocardial late gadolinium enhancement (LGE), 15 (35%) had findings of LGE with LVEF > 55% and 13 (30%) had LGE with LVEF < 55%. The magnitude of the peak basal circumferential strain, basal radial strain, and basal surface area strain were all significantly decreased in DMD patients relative to healthy controls (p < 0.001) with AUC values of 0.80, 0.89, and 0.84 respectively for peak strain and 0.96, 0.91, and 0.98 respectively for systolic strain rate. Peak basal radial strain, basal radial systolic strain rate, and basal circumferential systolic strain rate magnitude values were also significantly decreased in mild CMP (No LGE, LVEF > 55%) compared to a healthy control group (p < 0.001 for all). Surface area strain significantly correlated with LVEF and extracellular volume (ECV) respectively in the basal (rho = - 0.45, 0.40), mid (rho = - 0.46, 0.46), and apical (rho = - 0.42, 0.47) regions.
Conclusion: Strain analysis of 3D cine CMR images in DMD CMP patients generates localized kinematic parameters that strongly differentiate disease from control and correlate with LVEF and ECV.
Keywords: 3D; 4D; Cardiac biomechanics; Cardiac magnetic resonance; Cardiomyopathy; Duchenne muscular dystrophy; Strain.
© 2023. The Author(s).
Conflict of interest statement
The authors have no competing interests related to publication of this work.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

