Gold Nanoparticles Conjugated with Dendrigraft Poly-L-lysine and Folate-Targeted Poly(ethylene glycol) for siRNA Delivery to Prostate cancer
- PMID: 36793347
- PMCID: PMC9925352
- DOI: 10.7150/ntno.79050
Gold Nanoparticles Conjugated with Dendrigraft Poly-L-lysine and Folate-Targeted Poly(ethylene glycol) for siRNA Delivery to Prostate cancer
Abstract
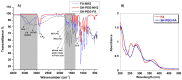
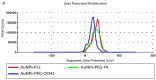
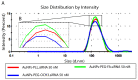
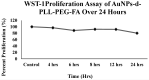
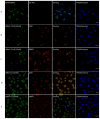
Dendrigraft Poly-L-Lysine (d-PLL) coated gold nanoparticles (AuNPs) were synthesized by reducing Tetrachloroauric acid with ascorbic acid in the presence of d-PLL. AuNPs-d-PLL formed a stable colloidal solution that absorbs light at a maximum wavelength (λmax) centered at 570 nm as demonstrated by UV-visible (UV-Vis) spectroscopy. From Scanning Electron Microscopy (SEM) analysis, AuNPs-d-PLL were spherical in shape with a mean diameter of 128 ± 47 nm. Dynamic Light scattering (DLS) analysis of the colloidal solution exhibited one size distribution with a hydrodynamic diameter of about 131 nm (size distribution by intensity). Zeta potential (ξ) measurements revealed positively charged AuNPs-d-PLL with ξ about 32 mV, an indicator of high stability in an aqueous solution. The AuNPs-d-PLL was successfully modified with either thiolated poly (ethylene glycol) SH-PEG-OCH3 (Mw 5400 g mol-1) or folic acid-modified thiolated poly (ethylene glycol) SH-PEG-FA of similar molecular weight as demonstrated via DLS and Zeta potential measurements. Complexation of PEGylated AuNPs-d-PLL with siRNA was confirmed by DLS and gel electrophoresis. Finally, we analyzed the functionalization of our nanocomplexes with folic acid via targeted cellular uptake to prostate cancer cells using flow cytometry and LSM imaging. Our findings implicate the broader applicability of folate-PEGylated AuNPs in siRNA-based therapeutics against prostate cancer and perhaps other types of cancer.
Keywords: cancer; gold nanoparticles; polymers; siRNA delivery; stabilization.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures







References
-
- Rahme K, Guo J, Biswas S, O'Driscoll CM, Holmes JD. Branched PEI Capped Gold Nanoparticles in Water for siRNA Delivery to Cancer Cells. Advanced Materials: TechConnect Briefs. 2017. pp. 159–62.
-
- Chanda N, Kan P, Watkinson LD, Shukla R, Zambre A, Carmack TL. et al. Radioactive gold nanoparticles in cancer therapy: therapeutic efficacy studies of GA-198AuNP nanoconstruct in prostate tumor-bearing mice. Nanomedicine: nanotechnology, biology, and medicine. 2010;6:201–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

