Syntheses and crystal structures of two di-naph-tho[2,1- d:1',2'- f][1,3]dithiepine atropisomers
- PMID: 36793408
- PMCID: PMC9912471
- DOI: 10.1107/S2056989023000476
Syntheses and crystal structures of two di-naph-tho[2,1- d:1',2'- f][1,3]dithiepine atropisomers
Abstract
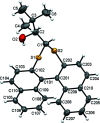
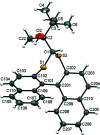
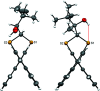
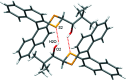
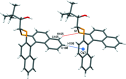
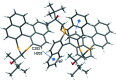
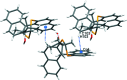
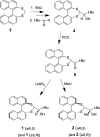
The closely related title compounds, 1-(di-naphtho-[2,1-d:1',2'-f][1,3]dithiepin-4-yl)-2,2-di-methyl-propan-1-ol, C26H24OS2, 1 and 2-(di-naphtho-[2,1-d:1',2'-f][1,3]dithiepin-4-yl)-3,3-di-methyl-butan-2-ol, C27H26OS2, 2, both comprise an atrop-isomeric binaphthyl di-thio-acetal unit substituted at the methyl-ene carbon atom with a chiral neopentyl alcohol grouping. The overall stereochemistry of the racemate in each case is defined as aS,R and aR,S. In 1, the hydroxyl group generates inversion dimers via pairwise inter-molecular O-H⋯S hydrogen bonds whereas in 2, the O-H⋯S link is intra-molecular. Weak C-H⋯π inter-actions link the mol-ecules into extended arrays in both structures.
Keywords: C—H⋯π contacts; asymmetric synthesis; atropisomer; binaphthalene dithiol; crystal structure; hydrogen bonds.
© Beare et al. 2023.
Figures
References
-
- Bruker (1998). SMART and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cheng, J. K., Xiang, S.-H., Li, S., Ye, L. & Tan, B. (2021). Chem. Rev. 121, 4805–4902. - PubMed
-
- Delogu, G., De Lucchi, O., Maglioli, P. & Valle, G. (1991). J. Org. Chem. 56, 4467–4473.
LinkOut - more resources
Full Text Sources
Research Materials
