Macrocyclic Retinoic Acid Receptor-Related Orphan Receptor C2 Inverse Agonists
- PMID: 36793423
- PMCID: PMC9923832
- DOI: 10.1021/acsmedchemlett.2c00500
Macrocyclic Retinoic Acid Receptor-Related Orphan Receptor C2 Inverse Agonists
Abstract
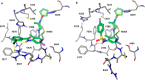
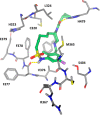
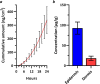
Macrocyclic retinoic acid receptor-related orphan receptor C2 (RORC2) inverse agonists have been designed with favorable properties for topical administration. Inspired by the unanticipated bound conformation of an acyclic sulfonamide-based RORC2 ligand from cocrystal structure analysis, macrocyclic linker connections between the halves of the molecule were explored. Further optimization of analogues was accomplished to maximize potency and refine physiochemical properties (MW, lipophilicity) best suited for topical application. Compound 14 demonstrated potent inhibition of interleukin-17A (IL-17A) production by human Th17 cells and in vitro permeation through healthy human skin achieving high total compound concentration in both skin epidermis and dermis layers.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Langley R. G.; Elewski B. E.; Lebwohl M.; Reich K.; Griffiths C. E. M.; Papp K.; Puig L.; Nakagawa H.; Spelman L.; Sigurgeirsson B.; Rivas E.; Tsai T.-F.; Wasel N.; Tyring S.; Salko T.; Hampele I.; Notter M.; Karpov A.; Helou S.; Papavassilis C. Secukinumab in plaque psoriasis – Results of two phase 3 trials. N. Engl. J. Med. 2014, 371, 326–338. 10.1056/NEJMoa1314258. - DOI - PubMed
-
- Griffiths C. E. M.; Reich K.; Lebwohl M.; van de Kerkhof P.; Paul C.; Menter A.; Cameron G. S.; Erickson J.; Zhang L.; Secrest R. J.; Ball S.; Braun D. K.; Osuntokun O. O.; Heffernan M. P.; Nickoloff B. J.; Papp K. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015, 386, 541–551. 10.1016/S0140-6736(15)60125-8. - DOI - PubMed
-
- Lebwohl M.; Strober B.; Menter A.; Gordon K.; Weglowska J.; Puig L.; Papp K.; Spelman L.; Toth D.; Kerdel F.; Armstrong A. W.; Stingl G.; Kimball A. B.; Bachelez H.; Wu J. J.; Crowley J.; Langley R. G.; Blicharski T.; Paul C.; Lacour J.-P.; Tyring S.; Kircik L.; Chimenti S.; Duffin K. C.; Bagel J.; Koo J.; Aras G.; Li J.; Song W.; Milmont C. E.; Shi Y.; Erondu N.; Klekotka P.; Kotzin B.; Nirula A. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N. Engl. J. Med. 2015, 373, 1318–1328. 10.1056/NEJMoa1503824. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

