Cancer-associated fibroblasts: The chief architect in the tumor microenvironment
- PMID: 36793444
- PMCID: PMC9923123
- DOI: 10.3389/fcell.2023.1089068
Cancer-associated fibroblasts: The chief architect in the tumor microenvironment
Abstract
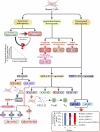
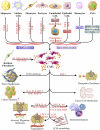
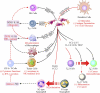
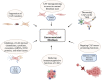
Stromal heterogeneity of tumor microenvironment (TME) plays a crucial role in malignancy and therapeutic resistance. Cancer-associated fibroblasts (CAFs) are one of the major players in tumor stroma. The heterogeneous sources of origin and subsequent impacts of crosstalk with breast cancer cells flaunt serious challenges before current therapies to cure triple-negative breast cancer (TNBC) and other cancers. The positive and reciprocal feedback of CAFs to induce cancer cells dictates their mutual synergy in establishing malignancy. Their substantial role in creating a tumor-promoting niche has reduced the efficacy of several anti-cancer treatments, including radiation, chemotherapy, immunotherapy, and endocrine therapy. Over the years, there has been an emphasis on understanding CAF-induced therapeutic resistance in order to enhance cancer therapy results. CAFs, in the majority of cases, employ crosstalk, stromal management, and other strategies to generate resilience in surrounding tumor cells. This emphasizes the significance of developing novel strategies that target particular tumor-promoting CAF subpopulations, which will improve treatment sensitivity and impede tumor growth. In this review, we discuss the current understanding of the origin and heterogeneity of CAFs, their role in tumor progression, and altering the tumor response to therapeutic agents in breast cancer. In addition, we also discuss the potential and possible approaches for CAF-mediated therapies.
Keywords: CAF; breast cancer; heterogeneity; targeting; tumor microenvironment.
Copyright © 2023 Sarkar, Nguyen, Gundre, Ogunlusi, El-Sobky, Giri and Sarkar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

