Exploring Predictive Risk Factors of Infusion Reactions with First Pertuzumab Administration in HER2-positive Breast Cancer Patients: A Single Institution Experience
- PMID: 36793527
- PMCID: PMC9908404
- DOI: 10.31662/jmaj.2022-0132
Exploring Predictive Risk Factors of Infusion Reactions with First Pertuzumab Administration in HER2-positive Breast Cancer Patients: A Single Institution Experience
Abstract
Introduction: Pertuzumab and trastuzumab are monoclonal antibodies used for treating HER2-positive breast cancer. These anti-HER2 antibodies may induce infusion reactions (IR), mainly upon first administration. We investigated factors predicting IR in the initial pertuzumab treatment for HER2-positive breast cancer.
Methods: We retrospectively reviewed the medical records of 57 patients who first received pertuzumab-containing treatment in our hospital from January 2014 to February 2021. The frequency of IR during or immediately after pertuzumab administration was examined. We also analyzed patient characteristics that may represent possible risk factors for IR.
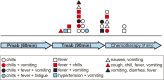
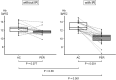
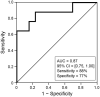
Results: The incidence rate of IR was 44% (25/57). Red blood cell count (P < 0.001), hemoglobin (Hb) concentration (P = 0.0011), and hematocrit (P < 0.001) immediately before pertuzumab administration were significantly lower in patients with IR than in those without. In patients with IR, erythrocyte levels immediately before pertuzumab treatment were significantly lower than baseline when having received anthracycline-containing chemotherapy within three months. Logistic regression analysis showed that a decrease in Hb levels was a significant risk factor for IR (log odds ratio = -17). According to the receiver-operating characteristic analysis, a 10% decrease in Hb after anthracycline-containing treatment was the best cut-off value for predicting IR (sensitivity: 88%; specificity: 77%; area under the curve: 0.87).
Conclusions: Our study showed a higher incidence of IR after pertuzumab treatment than in clinical trials. There was a strong association between IR occurrence and erythrocyte levels lower than baseline in the group that received anthracycline-containing chemotherapy immediately before.
Keywords: HER2; breast cancer; infusion reactions; pertuzumab; risk factor.
Copyright © Japan Medical Association.
Conflict of interest statement
None
Figures
Similar articles
-
Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): a randomised, open-label, multicentre, non-inferiority, phase 3 study.Lancet Oncol. 2021 Jan;22(1):85-97. doi: 10.1016/S1470-2045(20)30536-2. Epub 2020 Dec 21. Lancet Oncol. 2021. PMID: 33357420 Clinical Trial.
-
Pertuzumab and trastuzumab with or without metronomic chemotherapy for older patients with HER2-positive metastatic breast cancer (EORTC 75111-10114): an open-label, randomised, phase 2 trial from the Elderly Task Force/Breast Cancer Group.Lancet Oncol. 2018 Mar;19(3):323-336. doi: 10.1016/S1470-2045(18)30083-4. Epub 2018 Feb 9. Lancet Oncol. 2018. PMID: 29433963 Clinical Trial.
-
Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial.Lancet Oncol. 2018 Jan;19(1):115-126. doi: 10.1016/S1470-2045(17)30716-7. Epub 2017 Nov 23. Lancet Oncol. 2018. PMID: 29175149 Clinical Trial.
-
Pertuzumab for the treatment of human epidermal growth factor receptor type 2-positive metastatic breast cancer.Am J Health Syst Pharm. 2013 Sep 15;70(18):1579-87. doi: 10.2146/ajhp120735. Am J Health Syst Pharm. 2013. PMID: 23988598 Review.
-
Use of pertuzumab for the treatment of HER2-positive metastatic breast cancer.Adv Ther. 2013 Jul;30(7):645-58. doi: 10.1007/s12325-013-0043-2. Epub 2013 Jul 24. Adv Ther. 2013. PMID: 23881722 Review.
References
-
- Moelans CB, de Weger RA, Van der Wall E, et al. Current technologies for HER2 testing in breast cancer. Crit Rev Oncol Hematol. 2011;80(3):380-92. - PubMed
-
- Asselin B. Immunology of infusion reactions in the treatment of patients with acute lymphoblastic leukemia. Future Oncol. 2016;12(13):1609-21. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
