First-line programmed death-1 inhibitor treatment for locoregionally advanced or metastatic cutaneous squamous cell carcinoma - A real-world experience from Israel
- PMID: 36793605
- PMCID: PMC9924127
- DOI: 10.3389/fonc.2023.1117804
First-line programmed death-1 inhibitor treatment for locoregionally advanced or metastatic cutaneous squamous cell carcinoma - A real-world experience from Israel
Abstract
Objective: Cutaneous squamous cell carcinoma (cSCC) is the second most common non-melanoma skin cancer worldwide. It is usually treated surgically, with very high cure rates. However, in 3%-7% of cases, cSCC metastasizes to lymph nodes or distant organs. Many of the affected patients are elderly with comorbidities who are not candidates for standard-of-care curative-intent treatment with surgery and/or radio-/chemotherapy. Immune checkpoint inhibitors, which target programmed cell death protein 1 (PD-1) pathways, have recently emerged as a potent therapeutic option. The present report presents the Israeli experience with PD-1 inhibitors for the treatment of loco-regionally advanced or metastatic cSCC in a diverse and elderly population, with or without the addition of radiotherapy.
Material and methods: The databases of two university medical centers were retrospectively searched for patients with cSCC treated with the PD-1 inhibitors cemiplimab or pembrolizumab between January 2019 and May 2022. Data on baseline, disease-related, treatment-related, and outcome parameters were collected and analyzed.
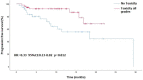
Results: The cohort included 102 patients of a median age 78.5 years. Evaluable response data were available for 93. The overall response rate was 80.6%: complete response in 42 patients (45.2%) and partial response in 33 (35.5%). Stable disease was recorded in 7 (7.5%) and progressive disease in 11 (11.8%). Median progression-free survival was 29.5 months. Radiotherapy was administered to the target lesion during PD-1 treatment in 22.5% of patients. mPFS was not significantly different in patients who treated with RT than patients how did not (NR vs 18.4 months, HR=0.93, 95%CI: 0.39 - 2.17, p<0.859). Any-grade toxicity was recorded in 57 patients (55%), including grade ‗3 in 25, of whom 5 (5% of cohort) died. Compared to toxicity-free patients, patients with drug toxicity had better progression-free survival (18.4 months vs not reached, HR=0.33, 95% CI: 0.13-0.82, p=0.012) and higher overall response rate (87% vs 71.8%, p=0.06).
Conclusion: This retrospective real-world study showed that PD-1 inhibitors were effective in the treatment of locally advanced or metastatic cSCC and appeared to be amenable for use in elderly or fragile patients with comorbidities. However, the high toxicity warrants consideration against other modalities. Induction or consolidation radiotherapy may improve the results. These findings need to be corroborated in a prospective trial.
Keywords: PD-1 inhibitor; cemiplimab; cutaneous squamous cell carcinoma (cSCC); pembrolizumab; radiotherapy; real-world experience.
Copyright © 2023 Averbuch, Salman, Shtamper, Doweck, Popovtzer, Markel, Hendler, Finkel, Moore, Fenig, Taha, Mhameed, Kurman and Billan.
Conflict of interest statement
GM received a research grant from Sanofi. He is also a consultant advisory board and received honoraria. IF received honoraria from Sanofi and from MSD. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor GB-S declared a shared parent affiliation with the authors SS, ID, and SB at the time of review.
Figures
References
-
- Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (Keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol (2015) 151(10):1081–6. - PubMed
-
- Stratigos A, Garbe C, Lebbe C, Malvehy J, Del Marmol V, Pehamberger H, et al. . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer (2015) 51(14):1989–2007. - PubMed
-
- Gül Ü, Kiliç A. Squamous cell carcinoma developing on burn scar. Ann Plast Surg (2006) 56(4):406–8. - PubMed
-
- Leonardi-Bee J, Ellison T, Bath-Hextall F. Smoking and the risk of non-melanoma skin cancer: Systematic review and meta-analysis. Arch Dermatol (2012) 148(8):939–46. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials