Low-dose X-ray radiodynamic therapy solely based on gold nanoclusters for efficient treatment of deep hypoxic solid tumors combined with enhanced antitumor immune response
- PMID: 36793856
- PMCID: PMC9925321
- DOI: 10.7150/thno.78649
Low-dose X-ray radiodynamic therapy solely based on gold nanoclusters for efficient treatment of deep hypoxic solid tumors combined with enhanced antitumor immune response
Abstract
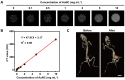
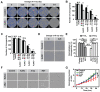
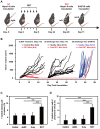
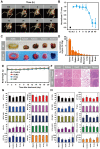
Background: Radiodynamic therapy (RDT) is an emerging novel anti-cancer treatment based on the generation of cytotoxic reactive oxygen species (ROS) at the lesion site following the interaction between low-dose X-ray and a photosensitizer (PS) drug. For a classical RDT, scintillator nanomaterials loaded with traditional PSs are generally involved to generate singlet oxygen (1O2). However, this scintillator-mediated strategy generally suffers from insufficient energy transfer efficiency and the hypoxic tumor microenvironment, and finally severely impedes the efficacy of RDT. Methods: Gold nanoclusters were irradiated by low dose of X-ray (called RDT) to investigate the production of ROS, killing efficiency of cell level and living body level, antitumor immune mechanism and biosafety. Results: A novel dihydrolipoic acid coated gold nanoclusters (AuNC@DHLA) RDT, without additional scintillator or photosensitizer assisted, has been developed. In contrast to scintillator-mediated strategy, AuNC@DHLA can directly absorb the X-ray and exhibit excellent radiodynamic performance. More importantly, the radiodynamic mechanism of AuNC@DHLA involves electron-transfer mode resulting in O2 -• and HO•, and excess ROS has been generated even under hypoxic conditions. Highly efficient in vivo treatment of solid tumors had been achieved via only single drug administration and low-dose X-ray radiation. Interestingly, enhanced antitumor immune response was involved, which could be effective against tumor recurrence or metastasis. Negligible systemic toxicity was also observed as a consequence of the ultra-small size of AuNC@DHLA and rapid clearance from body after effective treatment. Conclusions: Highly efficient in vivo treatment of solid tumors had been achieved, enhanced antitumor immune response and negligible systemic toxicity were observed. Our developed strategy will further promote the cancer therapeutic efficiency under low dose X-ray radiation and hypoxic conditions, and bring hope for clinical cancer treatment.
Keywords: antitumor immune; gold nanoclusters; radiodynamic therapy; solid tumor.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–7. - PubMed
-
- Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17:657–74. - PubMed
-
- Hu J, Tang Ya, Elmenoufy AH, Xu H, Cheng Z, Yang X. Nanocomposite-based photodynamic therapy strategies for deep tumor treatment. Small. 2015;11:5860–87. - PubMed
-
- Fan W, Huang P, Chen X. Overcoming the Achilles' heel of photodynamic therapy. Chem Soc Rev. 2016;45:6488–519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

