Saliva-based microfluidic point-of-care diagnostic
- PMID: 36793864
- PMCID: PMC9925318
- DOI: 10.7150/thno.78872
Saliva-based microfluidic point-of-care diagnostic
Abstract
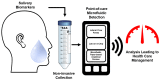
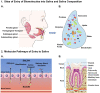
There has been a long-standing interest in point-of-care (POC) diagnostics as a tool to improve patient care because it can provide rapid, actionable results near the patient. Some of the successful examples of POC testing include lateral flow assays, urine dipsticks, and glucometers. Unfortunately, POC analysis is somewhat limited by the ability to manufacture simple devices to selectively measure disease specific biomarkers and the need for invasive biological sampling. Next generation POCs are being developed that make use of microfluidic devices to detect biomarkers in biological fluids in a non-invasive manner, addressing the above-mentioned limitations. Microfluidic devices are desirable because they can provide the ability to perform additional sample processing steps not available in existing commercial diagnostics. As a result, they can provide more sensitive and selective analysis. While most POC methods make use of blood or urine as a sample matrix, there has been a growing push to use saliva as a diagnostic medium. Saliva represents an ideal non-invasive biofluid for detecting biomarkers because it is readily available in large quantities and analyte levels reflect those in blood. However, using saliva in microfluidic devices for POC diagnostics is a relatively new and an emerging field. The overarching aim of this review is to provide an update on recent literature focused on the use of saliva as a biological sample matrix in microfluidic devices. We will first cover the characteristics of saliva as a sample medium and then review microfluidic devices that are developed for the analysis of salivary biomarkers.
Keywords: Diagnostics; Saliva; lab-on-chip; microfluidic.; point-of-care.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Integrated microfluidic platform for oral diagnostics.Ann N Y Acad Sci. 2007 Mar;1098:362-74. doi: 10.1196/annals.1384.004. Ann N Y Acad Sci. 2007. PMID: 17435142 Free PMC article. Review.
-
Lab-on-a-Disc for Point-of-Care Infection Diagnostics.Acc Chem Res. 2021 Oct 5;54(19):3643-3655. doi: 10.1021/acs.accounts.1c00367. Epub 2021 Sep 13. Acc Chem Res. 2021. PMID: 34516092
-
A critical insight into the development pipeline of microfluidic immunoassay devices for the sensitive quantitation of protein biomarkers at the point of care.Analyst. 2017 Mar 13;142(6):858-882. doi: 10.1039/c6an02445a. Analyst. 2017. PMID: 28217778 Review.
-
Translational and clinical applications of salivary diagnostics.Adv Dent Res. 2011 Oct;23(4):375-80. doi: 10.1177/0022034511420434. Adv Dent Res. 2011. PMID: 21917748 Free PMC article.
-
Point-of-care platforms for salivary diagnostics.Chin J Dent Res. 2012;15(1):7-15. Chin J Dent Res. 2012. PMID: 22866276
Cited by
-
Salivary Biomarkers: Noninvasive Ways for Diagnosis of Parkinson's Disease.Neurol Res Int. 2023 Jul 3;2023:3555418. doi: 10.1155/2023/3555418. eCollection 2023. Neurol Res Int. 2023. PMID: 37434876 Free PMC article. Review.
-
From Body Monitoring to Biomolecular Sensing: Current Progress and Future Perspectives of Triboelectric Nanogenerators in Point-of-Care Diagnostics.Sensors (Basel). 2024 Jan 14;24(2):511. doi: 10.3390/s24020511. Sensors (Basel). 2024. PMID: 38257606 Free PMC article. Review.
-
Filtered Saliva for Rapid and Accurate Analyte Detection for POC Diagnostics.Diagnostics (Basel). 2024 May 24;14(11):1088. doi: 10.3390/diagnostics14111088. Diagnostics (Basel). 2024. PMID: 38893615 Free PMC article.
-
Artificial Intelligence in Regenerative Medicine: Applications and Implications.Biomimetics (Basel). 2023 Sep 20;8(5):442. doi: 10.3390/biomimetics8050442. Biomimetics (Basel). 2023. PMID: 37754193 Free PMC article. Review.
-
POC device for rapid oral pH determination based on a smartphone platform.Mikrochim Acta. 2024 Feb 14;191(3):134. doi: 10.1007/s00604-024-06227-1. Mikrochim Acta. 2024. PMID: 38353778 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

