Purification of Functional Gene Transfer Agents Using Two-Step Preparative Monolithic Chromatography
- PMID: 36793882
- PMCID: PMC9917305
- DOI: 10.1089/phage.2022.0035
Purification of Functional Gene Transfer Agents Using Two-Step Preparative Monolithic Chromatography
Abstract
Background: Gene transfer agents (GTAs) are phage-like particles that transfer cellular genomic DNA between cells. A hurdle faced in studying GTA function and interactions with cells is the difficulty in obtaining pure and functional GTAs from cultures.
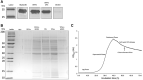
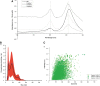
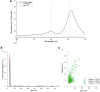
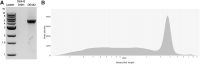
Materials and methods: We used a novel two-step method for purification of GTAs from R. capsulatus by monolithic chromatography.
Results: Our efficient and simple process had advantages compared to previous approaches. The purified GTAs retained gene transfer activity and the packaged DNA could be used for further studies.
Conclusions: This method is applicable to GTAs produced by other species and small phages, and could be useful for therapeutic applications.
Keywords: Convective Interaction Media®; Rhodobacter capsulatus; gene transfer; monolithic separations; nanoparticle tracking analysis; phage purification.
Copyright 2022, Mary Ann Liebert, Inc., publishers.
Conflict of interest statement
No competing financial interests exist.
Figures


References
LinkOut - more resources
Full Text Sources
