Current understanding of metal-dependent amyloid-β aggregation and toxicity
- PMID: 36794021
- PMCID: PMC9906324
- DOI: 10.1039/d2cb00208f
Current understanding of metal-dependent amyloid-β aggregation and toxicity
Abstract
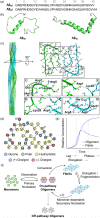
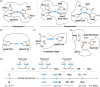
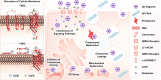
The discovery of effective therapeutics targeting amyloid-β (Aβ) aggregates for Alzheimer's disease (AD) has been very challenging, which suggests its complicated etiology associated with multiple pathogenic elements. In AD-affected brains, highly concentrated metals, such as copper and zinc, are found in senile plaques mainly composed of Aβ aggregates. These metal ions are coordinated to Aβ and affect its aggregation and toxicity profiles. In this review, we illustrate the current view on molecular insights into the assembly of Aβ peptides in the absence and presence of metal ions as well as the effect of metal ions on their toxicity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Sevigny J. Chiao P. Bussière T. Weinreb P. H. Williams L. Maier M. Dunstan R. Salloway S. Chen T. Ling Y. O'Gorman J. Qian F. Arastu M. Li M. Chollate S. Brennan M. S. Quintero-Monzon O. Scannevin R. H. Arnold H. M. Engber T. Rhodes K. Ferrero J. Hang Y. Mikulskis A. Grimm J. Hock C. Nitsch R. M. Sandrock A. Nature. 2016;537:50–56. doi: 10.1038/nature19323. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

