Investigation of metal modulation of oxytocin structure receptor-mediated signaling
- PMID: 36794023
- PMCID: PMC9906307
- DOI: 10.1039/d2cb00225f
Investigation of metal modulation of oxytocin structure receptor-mediated signaling
Abstract
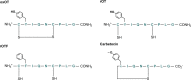
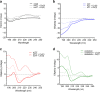
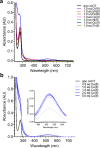
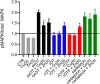
Oxytocin is a 9-amino acid peptide hormone. Since its discovery in 1954, it has most commonly been studied in relation to its role in stimulating parturition and lactation. However, it is now known that oxytocin has a widely diverse set of functions throughout the body including neuromodulation, bone growth, and inflammation. Previous research has suggested that divalent metal ions may be required for oxytocin activity, but the exact metal species and specific pathways have yet to be fully elucidated. In this work, we focus on characterizing copper and zinc bound forms of oxytocin and related analogs through far-UV circular dichroism. We report that Cu(ii) and Zn(ii) bind uniquely to oxytocin and all analogs investigated. Furthermore, we investigate how these metal bound forms may affect downstream signaling of MAPK activation upon receptor binding. We find that both Cu(ii) and Zn(ii) bound oxytocin attenuates the activation of the MAPK pathway upon receptor binding relative to oxytocin alone. Interestingly, we observed that Zn(ii) bound forms of linear oxytocin facilitate increased MAPK signaling. This study lays the foundation for future work on elucidating the metal effects on oxytocin's diverse bioactivity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

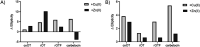
Similar articles
-
A zinc selective oxytocin based biosensor.J Mater Chem B. 2020 Jan 7;8(1):155-160. doi: 10.1039/c9tb01932d. Epub 2019 Nov 29. J Mater Chem B. 2020. PMID: 31782469
-
Copper-zinc cross-modulation in prion protein binding.Eur Biophys J. 2014 Dec;43(12):631-42. doi: 10.1007/s00249-014-0993-6. Epub 2014 Nov 14. Eur Biophys J. 2014. PMID: 25395329 Free PMC article.
-
Neuromodulatory functions exerted by oxytocin on different populations of hippocampal neurons in rodents.Front Cell Neurosci. 2023 Feb 2;17:1082010. doi: 10.3389/fncel.2023.1082010. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36816855 Free PMC article. Review.
-
On the possible roles of N-terminal His-rich domains of Cu,Zn SODs of some Gram-negative bacteria.J Inorg Biochem. 2012 Jan;106(1):10-8. doi: 10.1016/j.jinorgbio.2011.09.029. Epub 2011 Sep 29. J Inorg Biochem. 2012. PMID: 22105012
-
Clinical Experiences and Mechanism of Action with the Use of Oxytocin Injection at Parturition in Domestic Animals: Effect on the Myometrium and Fetuses.Animals (Basel). 2023 Feb 20;13(4):768. doi: 10.3390/ani13040768. Animals (Basel). 2023. PMID: 36830555 Free PMC article. Review.
Cited by
-
Structure and activity of conopressins: insights into in silico oxytocin/V2 receptor interactions, anti-inflammatory potential, and behavioural studies.RSC Med Chem. 2025 Jul 4. doi: 10.1039/d5md00288e. Online ahead of print. RSC Med Chem. 2025. PMID: 40726970 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

