Sustainable and practical formation of carbon-carbon and carbon-heteroatom bonds employing organo-alkali metal reagents
- PMID: 36794178
- PMCID: PMC9906645
- DOI: 10.1039/d2sc05475b
Sustainable and practical formation of carbon-carbon and carbon-heteroatom bonds employing organo-alkali metal reagents
Abstract
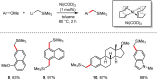
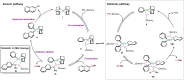
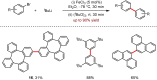
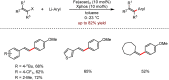
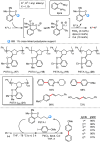
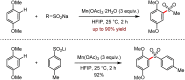
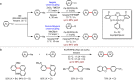
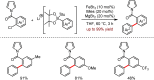
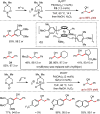
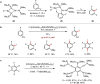
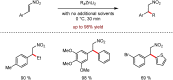
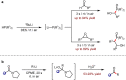
Metal-catalysed cross-coupling reactions are amongst the most widely used methods to directly construct new bonds. In this connection, sustainable and practical protocols, especially transition metal-catalysed cross-coupling reactions, have become the focus in many aspects of synthetic chemistry due to their high efficiency and atom economy. This review summarises recent advances from 2012 to 2022 in the formation of carbon-carbon bonds and carbon-heteroatom bonds by employing organo-alkali metal reagents.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



















Similar articles
-
Photocatalytic Activation of Less Reactive Bonds and Their Functionalization via Hydrogen-Evolution Cross-Couplings.Acc Chem Res. 2018 Oct 16;51(10):2512-2523. doi: 10.1021/acs.accounts.8b00267. Epub 2018 Oct 3. Acc Chem Res. 2018. PMID: 30280898
-
Recent advances in asymmetric borylation by transition metal catalysis.Chem Soc Rev. 2021 Nov 29;50(23):13129-13188. doi: 10.1039/d0cs00843e. Chem Soc Rev. 2021. PMID: 34709239 Review.
-
Progress in copper-catalysed/mediated intramolecular dehydrogenative coupling.Org Biomol Chem. 2023 Jan 4;21(2):237-251. doi: 10.1039/d2ob01796b. Org Biomol Chem. 2023. PMID: 36448561 Review.
-
The promise and challenge of iron-catalyzed cross coupling.Acc Chem Res. 2008 Nov 18;41(11):1500-11. doi: 10.1021/ar800039x. Acc Chem Res. 2008. PMID: 18588321
-
Cross-Dehydrogenative Coupling Reactions Between C(sp)-H and X-H (X = N, P, S, Si, Sn) Bonds: An Environmentally Benign Access to Heteroatom-Substituted Alkynes.Top Curr Chem (Cham). 2019 Jul 4;377(4):20. doi: 10.1007/s41061-019-0245-4. Top Curr Chem (Cham). 2019. PMID: 31273478 Review.
Cited by
-
The Versatile and Strategic O-Carbamate Directed Metalation Group in the Synthesis of Aromatic Molecules: An Update.Chem Rev. 2024 Jun 26;124(12):7731-7828. doi: 10.1021/acs.chemrev.3c00923. Epub 2024 Jun 12. Chem Rev. 2024. PMID: 38864673 Free PMC article. Review.
-
Synthesis and Reactivity of Dipalladated Derivatives of Terephthalaldehyde.Organometallics. 2024 Jul 24;43(15):1647-1657. doi: 10.1021/acs.organomet.4c00231. eCollection 2024 Aug 12. Organometallics. 2024. PMID: 39148863 Free PMC article.
-
A fruitful century for the scalable synthesis and reactions of biphenyl derivatives: applications and biological aspects.RSC Adv. 2023 Jun 16;13(27):18262-18305. doi: 10.1039/d3ra03531j. eCollection 2023 Jun 15. RSC Adv. 2023. PMID: 37333795 Free PMC article. Review.
-
Harnessing Organopotassium Reagents for Cross-Coupling with YPhos-Pd Catalysts: Opportunities, Applications, and Challenges.J Am Chem Soc. 2025 Feb 12;147(6):5417-5425. doi: 10.1021/jacs.4c18073. Epub 2025 Feb 2. J Am Chem Soc. 2025. PMID: 39893653 Free PMC article.
-
A silylboronate-mediated strategy for cross-coupling of alkyl fluorides with aryl alkanes: mechanistic insights and scope expansion.Chem Sci. 2024 Oct 1;15(42):17418-24. doi: 10.1039/d4sc04357j. Online ahead of print. Chem Sci. 2024. PMID: 39364067 Free PMC article.
References
-
-
For recent reviews, see:
- Fricke C. Sperger T. Mendel M. Schoenebeck F. Angew. Chem., Int. Ed. 2021;60:3355–3366. doi: 10.1002/anie.202011825. - DOI - PMC - PubMed
- Shaughnessy K. H. Isr. J. Chem. 2020;60:180–194. doi: 10.1002/ijch.201900067. - DOI
- Gildner P. G. Colacot T. J. Organometallics. 2015;34:5497–5508. doi: 10.1021/acs.organomet.5b00567. - DOI
- Johansson Seechurn C. C. C. Kitching M. O. Colacot T. J. Snieckus V. Angew. Chem., Int. Ed. 2012;51:5062–5085. doi: 10.1002/anie.201107017. - DOI - PubMed
-
-
- Johansson Seechurn C. C. C. Kitching O. M. Colacot T. J. Snieckus V. Angew. Chem., Int. Ed. 2012;51:5062–5085. doi: 10.1002/anie.201107017. - DOI - PubMed
- Biffis A. Centomo P. Del Zotto A. Zecca M. Chem. Rev. 2018;118:2249–2295. doi: 10.1021/acs.chemrev.7b00443. - DOI - PubMed
- Christoffel F. Ward T. R. Catal. Lett. 2018;148:489–511. doi: 10.1007/s10562-017-2285-0. - DOI
- Roy D. Uzomi Y. Adv. Synth. Catal. 2018;360:602–605. doi: 10.1002/adsc.201700810. - DOI
- Ruiz-Castillo P. Buchwald S. L. Chem. Rev. 2016;116:12564–12649. doi: 10.1021/acs.chemrev.6b00512. - DOI - PMC - PubMed
-
- Schlosser M., Organometallics in Synthesis: A Manual, Wiley, 2nd edn, 2002
- Seyferth D. Organometallics. 2009;28:2–33. doi: 10.1021/om801047n. - DOI
- Nobis J. F. Moormeier L. F. Robinson R. E. Adv. Chem. 1959;23:63–68.
- Seyferth D. Organometallics. 2001;20:2940–2955. doi: 10.1021/om010439f. - DOI
- Gentner T. X. Mulvey R. E. Angew. Chem., Int. Ed. 2021;60:9247–9262. doi: 10.1002/anie.202010963. - DOI - PMC - PubMed
- https://www.rsc.org/periodic-table/, accessed 10 November 2022
- Chen Z. Yang H. Kang Z. Driess M. Menezes P. W. Adv. Mater. 2022;34:2108432. doi: 10.1002/adma.202108432. - DOI - PubMed
-
-
For recent reviews, see:
- Heravi M. M. Zadsirjan V. Hajiabbasi P. Habidi H. Chem. Mon. 2019;150:535–591. doi: 10.1007/s00706-019-2364-6. - DOI
- Qureshi Z. Toker C. Lautens M. Synthesis. 2017;49:1–16.
- Jana R. Pathak T. P. Sigman M. S. Chem. Rev. 2011;111:1417–1492. doi: 10.1021/cr100327p. - DOI - PMC - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources

