SUPERMAN strikes again in legumes
- PMID: 36794219
- PMCID: PMC9923009
- DOI: 10.3389/fpls.2023.1120342
SUPERMAN strikes again in legumes
Abstract
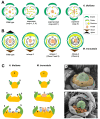
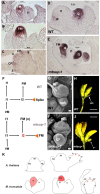
The SUPERMAN (SUP) gene was described in Arabidopsis thaliana over 30 years ago. SUP was classified as a cadastral gene required to maintain the boundaries between reproductive organs, thus controlling stamen and carpel number in flowers. We summarize the information on the characterization of SUP orthologs in plant species other than Arabidopsis, focusing on the findings for the MtSUP, the ortholog in the legume Medicago truncatula. M. truncatula has been widely used as a model system to study the distinctive developmental traits of this family of plants, such as the existence of compound inflorescence and complex floral development. MtSUP participates in the complex genetic network controlling these developmental processes in legumes, sharing conserved functions with SUP. However, transcriptional divergence between SUP and MtSUP provided context-specific novel functions for a SUPERMAN ortholog in a legume species. MtSUP controls the number of flowers per inflorescence and the number of petals, stamens and carpels regulating the determinacy of ephemeral meristems that are unique in legumes. Results obtained in M. truncatula provided new insights to the knowledge of compound inflorescence and flower development in legumes. Since legumes are valuable crop species worldwide, with high nutritional value and important roles in sustainable agriculture and food security, new information on the genetic control of their compound inflorescence and floral development could be used for plant breeding.
Keywords: Medicago truncatula; MtSUP; SUPERMAN; compound inflorescence; flower development; flower number; legumes.
Copyright © 2023 Rodas, Roque, Hamza, Gómez-Mena, Beltrán and Cañas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Benlloch R., D’Erfurth I., Ferrandiz C., Cosson V., Beltrán J. P., Cañas L. A., et al. . (2006). Isolation of mtpim proves Tnt1 a useful reverse genetics tool in Medicago truncatula and uncovers new aspects of AP1-like functions in legumes. Plant Physiol. 142, 972–983. doi: 10.1104/pp.106.083543 - DOI - PMC - PubMed
-
- Benlloch R., Navarro C., Beltrán J. P., Cañas L. A. (2003). Floral development of the model legume Medicago truncatula: Ontogeny studies as a tool to better characterize homeotic mutations. Sexual Plant Reprod. 15, 231–241. doi: 10.1007/s00497-002-0157-1 - DOI

