ATR Inhibitor Synergizes PARP Inhibitor Cytotoxicity in Homologous Recombination Repair Deficiency TK6 Cell Lines
- PMID: 36794257
- PMCID: PMC9925244
- DOI: 10.1155/2023/7891753
ATR Inhibitor Synergizes PARP Inhibitor Cytotoxicity in Homologous Recombination Repair Deficiency TK6 Cell Lines
Abstract
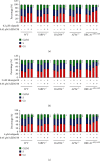
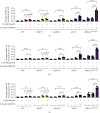
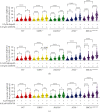
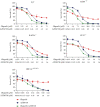
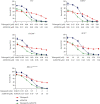
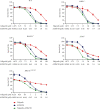
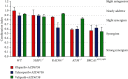
The inhibition of poly(ADP-ribose) polymerases (PARPs) and ataxia telangiectasia and Rad3-related (ATR) would be an alternative approach for cancer treatments. The aim of this study is to investigate the synergy of the different combinations of PARP inhibitors (olaparib, talazoparib, or veliparib) and ATR inhibitor AZD6738. A drug combinational synergy screen that combines olaparib, talazoparib, or veliparib with AZD6738 was performed to identify the synergistic interaction, and the combination index was calculated to verify synergy. TK6 isogenic cell lines with defects in different DNA repair genes were used as a model. Cell cycle analysis, micronucleus induction, and focus formation assays of serine-139 phosphorylation of the histone variant H2AX demonstrated that AZD6738 diminished G2/M checkpoint activation induced by PARP inhibitors and allowed DNA damage-containing cells to continue dividing, leading to greater increases in micronuclei as well as double-strand DNA breaks in mitotic cells. We also found that AZD6738 was likely to potentiate cytotoxicity of PARP inhibitors in homologous recombination repair deficiency cell lines. AZD6738 sensitized more genotypes of DNA repair-deficient cell lines to talazoparib than to olaparib and veliparib, respectively. The combinational approach of PARP and ATR inhibition to enhance response to PARP inhibitors could expand the utility of PARP inhibitors to cancer patients without BRCA1/2 mutations.
Copyright © 2023 Rakkreat Wikiniyadhanee et al.
Conflict of interest statement
The authors declare that there is no competing interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

