Extracellular vesicles in bacterial and fungal diseases - Pathogenesis to diagnostic biomarkers
- PMID: 36794396
- PMCID: PMC10012962
- DOI: 10.1080/21505594.2023.2180934
Extracellular vesicles in bacterial and fungal diseases - Pathogenesis to diagnostic biomarkers
Abstract
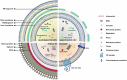
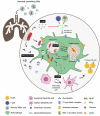
Intercellular communication among microbes plays an important role in disease exacerbation. Recent advances have described small vesicles, termed as "extracellular vesicles" (EVs), previously disregarded as "cellular dust" to be vital in the intracellular and intercellular communication in host-microbe interactions. These signals have been known to initiate host damage and transfer of a variety of cargo including proteins, lipid particles, DNA, mRNA, and miRNAs. Microbial EVs, referred to generally as "membrane vesicles" (MVs), play a key role in disease exacerbation suggesting their importance in pathogenicity. Host EVs help coordinate antimicrobial responses and prime the immune cells for pathogen attack. Hence EVs with their central role in microbe-host communication, may serve as important diagnostic biomarkers of microbial pathogenesis. In this review, we summarize current research regarding the roles of EVs as markers of microbial pathogenesis with specific focus on their interaction with host immune defence and their potential as diagnostic biomarkers in disease conditions.
Keywords: Biomarkers; extracellular vesicles; infection diagnosis; microbial pathogenesis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
