Monocyte chemoattractant protein 1 as a potential biomarker for immune checkpoint inhibitor-associated neurotoxicity
- PMID: 36794673
- PMCID: PMC10166892
- DOI: 10.1002/cam4.5695
Monocyte chemoattractant protein 1 as a potential biomarker for immune checkpoint inhibitor-associated neurotoxicity
Abstract
Background: Oncological patients can benefit substantially from treatment with immune checkpoint inhibitors (ICI). However, there is a growing awareness of immune-related adverse events (irAE). Especially ICI-mediated neurological adverse events (nAE(+)), are tough to diagnose and biomarkers to identify patients at risk are missing.
Methods: A prospective register with prespecified examinations was established for ICI treated patients in December 2019. At the time of data cut-off, 110 patients were enrolled and completed the clinical protocol. Herein, cytokines and serum neurofilament light chain (sNFL) from 21 patients were analyzed.
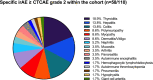
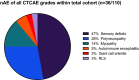
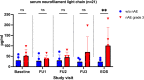
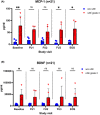
Results: nAE of any grade were observed in 31% of the patients (n = 34/110). In nAE(+) patients a significant increase in sNFL concentrations over time was observed. Patients with higher-grade nAE had significantly elevated serum-concentrations of monocyte chemoattractant protein 1 (MCP-1) and brain-derived neurotrophic factor (BDNF) at baseline compared to individuals without any nAE (p < 0.01 and p < 0.05).
Conclusion: Here, we identified nAE to occur more frequently than previously reported. Increase of sNFL during nAE confirms the clinical diagnosis of neurotoxicity and might be a suitable marker for neuronal damage associated with ICI therapy. Furthermore, MCP-1 and BDNF are potentially the first clinical-class nAE predictors for patients under ICI therapy.
Keywords: biomarker; immune-related adverse events; immunotherapy; neurotoxicity; serum neurofilament light chains.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Chan MM, Kefford RF, Carlino M, Clements A, Manolios N. Arthritis and tenosynovitis associated with the anti‐PD1 antibody pembrolizumab in metastatic melanoma. J Immunother. 2015;38:37‐39. - PubMed
-
- Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK‐3475; anti‐PD‐1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21:4286‐4293. - PubMed
-
- Bellmunt J, Bajorin DF. Pembrolizumab for advanced urothelial carcinoma. N Engl J Med. 2017;376:2304. - PubMed
-
- Kostine M, Rouxel L, Barnetche T, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer‐clinical aspects and relationship with tumour response: a single‐centre prospective cohort study. Ann Rheum Dis. 2018;77:393‐398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

