Prognostic analysis of three forms of Ki-67 in patients with breast cancer with non-pathological complete response before and after neoadjuvant systemic treatment
- PMID: 36794698
- PMCID: PMC10166904
- DOI: 10.1002/cam4.5693
Prognostic analysis of three forms of Ki-67 in patients with breast cancer with non-pathological complete response before and after neoadjuvant systemic treatment
Abstract
Background: Patients who do not achieve a pathological complete response (pCR) after neoadjuvant systemic treatment (NST) have a significantly worse prognosis. A reliable predictor of prognosis is required to further subdivide non-pCR patients. To date, the prognostic role in terms of disease-free survival (DFS) between the terminal index of Ki-67 after surgery (Ki-67T ) and the combination of the baseline Ki-67 at biopsy before NST (Ki-67B ) and the percentage change in Ki-67 before and after NST (Ki-67C ) has not been compared.
Aim: This study aimed to explore the most useful form or combination of Ki-67 that can provide prognostic information to non-pCR patients.
Patients and methods: We retrospectively reviewed 499 patients who were diagnosed with inoperable breast cancer between August 2013 and December 2020 and received NST with anthracycline plus taxane.
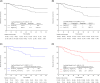
Results: Among all the patients, 335 did not achieve pCR (with a follow-up period of ≥1 year). The median follow-up duration was 36 months. The optimal cutoff value of Ki-67C to predict a DFS was 30%. A significantly worse DFS was observed in patients with a low Ki-67C (p < 0.001). In addition, the exploratory subgroup analysis showed relatively good internal consistency. Ki-67C and Ki-67T were considered as independent risk factors for DFS (both p < 0.001). The forecasting model combining Ki-67B and Ki-67C showed a significantly higher area under the curve at years 3 and 5 than Ki-67T (p = 0.029 and p = 0.022, respectively).
Conclusions: Ki-67C and Ki-67T were good independent predictors of DFS, whereas Ki-67B was a slightly inferior predictor. The combination of Ki-67B and Ki-67C is superior to Ki-67T for predicting DFS, especially at longer follow-ups. Regarding clinical application, this combination could be used as a novel indicator for predicting DFS to more clearly identify high-risk patients.
Keywords: breast cancer; cell cycle; neoadjuvant chemotherapy; prognosis.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Prognostic assessment of breast carcinoma submitted to neoadjuvant chemotherapy with pathological non-complete response.BMC Cancer. 2019 Jun 17;19(1):601. doi: 10.1186/s12885-019-5812-0. BMC Cancer. 2019. PMID: 31208353 Free PMC article. Clinical Trial.
-
Ki-67 as a controversial predictive and prognostic marker in breast cancer patients treated with neoadjuvant chemotherapy.Diagn Pathol. 2017 Feb 21;12(1):20. doi: 10.1186/s13000-017-0608-5. Diagn Pathol. 2017. PMID: 28222768 Free PMC article.
-
Neoadjuvant chemotherapy in triple-negative breast cancer: A multicentric retrospective observational study in real-life setting.J Cell Physiol. 2018 Mar;233(3):2313-2323. doi: 10.1002/jcp.26103. Epub 2017 Sep 27. J Cell Physiol. 2018. PMID: 28710865
-
Real-world data on breast pathologic complete response and disease-free survival after neoadjuvant chemotherapy for hormone receptor-positive, human epidermal growth factor receptor-2-negative breast cancer: a multicenter, retrospective study in China.World J Surg Oncol. 2022 Sep 29;20(1):326. doi: 10.1186/s12957-022-02787-9. World J Surg Oncol. 2022. PMID: 36175898 Free PMC article.
-
Pathological complete response and prognosis after neoadjuvant chemotherapy in patients with HER2-low breast cancer.Ann Diagn Pathol. 2023 Jun;64:152125. doi: 10.1016/j.anndiagpath.2023.152125. Epub 2023 Feb 17. Ann Diagn Pathol. 2023. PMID: 36822053
Cited by
-
Evaluation of the relationship between Ki67 expression level and neoadjuvant treatment response and prognosis in breast cancer based on the Neo-Bioscore staging system.Discov Oncol. 2023 Oct 24;14(1):190. doi: 10.1007/s12672-023-00809-w. Discov Oncol. 2023. PMID: 37875716 Free PMC article.
-
Pretreatment Circulating Albumin, Platelet, and RDW-SD Associated with Worse Disease-Free Survival in Patients with Breast Cancer.Breast Cancer (Dove Med Press). 2024 Jan 16;16:23-39. doi: 10.2147/BCTT.S443292. eCollection 2024. Breast Cancer (Dove Med Press). 2024. PMID: 38250195 Free PMC article.
References
-
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672‐2685. - PubMed
-
- van der Hage JA, van de Velde CJ, Julien JP, Tubiana‐Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of cancer trial 10902. J Clin Oncol. 2001;19(22):4224‐4237. - PubMed
-
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long‐term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164‐172. - PubMed
-
- Cortazar P, Geyer CE Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol. 2015;22(5):1441‐1446. - PubMed
-
- Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta‐analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 2012;48(18):3342–3354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

