The HOPX and BLBP landscape and gliogenic regions in developing human brain
- PMID: 36794762
- PMCID: PMC10273337
- DOI: 10.1111/joa.13844
The HOPX and BLBP landscape and gliogenic regions in developing human brain
Abstract
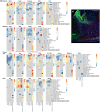
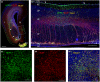
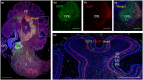
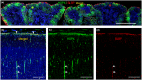
Outer radial glial cells (oRGs) give rise to neurons and glial cells and contribute to cell migration and expansion in developing neocortex. HOPX has been described as a marker of oRGs and possible actor in glioblastomas. Recent years' evidence points to spatiotemporal differences in brain development which may have implications for the classification of cell types in the central nervous system and understanding of a range of neurological diseases. Using the Human Embryonic/Fetal Biobank, Institute of Cellular and Molecular Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark, HOPX and BLBP immunoexpression was investigated in developing frontal, parietal, temporal and occipital human neocortex, other cortical areas and brain stem regions to interrogate oRG and HOPX regional heterogeneity. Furthermore, usage of high-plex spatial profiling (Nanostring GeoMx® DSP) was tested on the same material. HOPX marked oRGs in several human developing brain regions as well as cells in known gliogenic areas but did not completely overlap with BLBP or GFAP. Interestingly, limbic structures (e.g. olfactory bulb, indusium griseum, entorhinal cortex, fimbria) showed more intense HOPX immunoreactivity than adjacent neocortex and in cerebellum and brain stem, HOPX and BLBP seemed to stain different cell populations in cerebellar cortex and corpus pontobulbare. DSP screening of corresponding regions indicated differences in cell type composition, vessel density and presence of apolipoproteins within and across regions and thereby confirming the importance of acknowledging time and place in developmental neuroscience.
Keywords: BLBP; CNS; HOPX; fetal; human.
© 2023 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Figures





References
-
- Altman, J. & Bayer, S.A. (2002) Regional differences in the stratified transitional field and the honeycomb matrix of the developing human cerebral cortex. Journal of Neurocytology, 31, 613–632. - PubMed
-
- Barry, D.S. , Pakan, J.M. & Mcdermott, K.W. (2014) Radial glial cells: key organisers in CNS development. The International Journal of Biochemistry & Cell Biology, 46, 76–79. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

