Update on Measuring Ketones
- PMID: 36794812
- PMCID: PMC11089855
- DOI: 10.1177/19322968231152236
Update on Measuring Ketones
Abstract
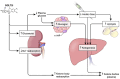
Ketone bodies are an energy substrate produced by the liver and used during states of low carbohydrate availability, such as fasting or prolonged exercise. High ketone concentrations can be present with insulin insufficiency and are a key finding in diabetic ketoacidosis (DKA). During states of insulin deficiency, lipolysis increases and a flood of circulating free fatty acids is converted in the liver into ketone bodies-mainly beta-hydroxybutyrate and acetoacetate. During DKA, beta-hydroxybutyrate is the predominant ketone in blood. As DKA resolves, beta-hydroxybutyrate is oxidized to acetoacetate, which is the predominant ketone in the urine. Because of this lag, a urine ketone test might be increasing even as DKA is resolving. Point-of-care tests are available for self-testing of blood ketones and urine ketones through measurement of beta-hydroxybutyrate and acetoacetate and are cleared by the US Food and Drug Administration (FDA). Acetone forms through spontaneous decarboxylation of acetoacetate and can be measured in exhaled breath, but currently no device is FDA-cleared for this purpose. Recently, technology has been announced for measuring beta-hydroxybutyrate in interstitial fluid. Measurement of ketones can be helpful to assess compliance with low carbohydrate diets; assessment of acidosis associated with alcohol use, in conjunction with SGLT2 inhibitors and immune checkpoint inhibitor therapy, both of which can increase the risk of DKA; and to identify DKA due to insulin deficiency. This article reviews the challenges and shortcomings of ketone testing in diabetes treatment and summarizes emerging trends in the measurement of ketones in the blood, urine, breath, and interstitial fluid.
Keywords: SGLT2 inhibitors; continuous ketone monitor; diabetes; diabetic ketoacidosis; insulin; ketones.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.M.B. has received research support, consulted, or has been on a scientific advisory board for Abbott Diabetes Care, Ascensia, Bigfoot Biomedical, CeQur, Dexcom, Hygieia, Insulet, Lilly, Medtronic, Novo Nordisk, Onduo, Roche Diabetes Care, Sanofi, Tandem Diabetes Care, United Healthcare, Vertex Pharmaceutical and Zealand Pharma. His technology research is funded in part by NIH/NIDDK and Helmsley Charitable Trust. R.M.B.’s employer, nonprofit HealthPartners Institute, contracts for his services, and no personal income goes to him. K.C. receives research support provided to her institution from Dexcom, Abbott, Medtronic, Altimmune, Lilly, Novo Nordisk, and Insulet and has received consulting fees from Dexcom. E.C. is an advisory board member and consultant for Novo Nordisk, Eli Lilly, Adocia, MannKind, Lexicon, Arecor. E.C. was also a speaker for Novo Nordisk. K.D. is the chair of the Joint British Diabetes Societies for Inpatient Care and has received speaker fees, travel, or taken part in advisory boards for AstraZeneca, Sanofi Diabetes, Boehringer Ingelheim, Lilly, and Novo Nordisk. J.L.S. has conducted clinical trials for Eli Lilly, Insulet, and Medtronic and has received in-kind support for research studies from Dexcom and Medtronic. She has consulted for Eli Lilly, Lexicon, Medtronic, and Sanofi. She has been a member of advisory boards for Bigfoot Biomedical, Cecelia Health, Eli Lilly, Insulet, the T1D Fund, and Vertex. G.E.U. reports research funds to Emory University from Astra Zeneca, Abbott, and Dexcom. The support from Astra Zeneca ended in 2022. D.C.K. is a consultant to EOFlow, Fractyl Health, Integrity, Lifecare, Rockley Photonics, and Thirdwayv. J.H., A.M.Y., and I.N. have nothing to disclose.
Figures


References
-
- Lohano PD, Ibrahim M, Raza SJ, Gowa M, Baloch SH. Comparing finger-stick βeta-hydroxybutyrate with dipstick urine tests in the detection of ketone bodies in the diagnosis of children with diabetic ketoacidosis. J Coll Physicians Surg Pak. 2022;32(4):483-486. doi: 10.29271/jcpsp.2022.04.483. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

