High Sox2 expression predicts taste lineage competency of lingual progenitors in vitro
- PMID: 36794954
- PMCID: PMC10112921
- DOI: 10.1242/dev.201375
High Sox2 expression predicts taste lineage competency of lingual progenitors in vitro
Abstract
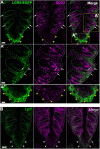
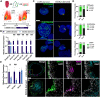
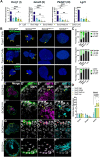
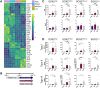
Taste buds on the tongue contain taste receptor cells (TRCs) that detect sweet, sour, salty, umami and bitter stimuli. Like non-taste lingual epithelium, TRCs are renewed from basal keratinocytes, many of which express the transcription factor SOX2. Genetic lineage tracing has shown that SOX2+ lingual progenitors give rise to both taste and non-taste lingual epithelium in the posterior circumvallate taste papilla (CVP) of mice. However, SOX2 is variably expressed among CVP epithelial cells, suggesting that their progenitor potential may vary. Using transcriptome analysis and organoid technology, we show that cells expressing SOX2 at higher levels are taste-competent progenitors that give rise to organoids comprising both TRCs and lingual epithelium. Conversely, organoids derived from progenitors that express SOX2 at lower levels are composed entirely of non-taste cells. Hedgehog and WNT/β-catenin are required for taste homeostasis in adult mice. However, manipulation of hedgehog signaling in organoids has no impact on TRC differentiation or progenitor proliferation. By contrast, WNT/β-catenin promotes TRC differentiation in vitro in organoids derived from higher but not low SOX2+ expressing progenitors.
Keywords: Adult stem cell; Organoid; Taste bud.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


Similar articles
-
SOX2 regulates homeostasis of taste bud cells and lingual epithelial cells in posterior tongue.PLoS One. 2020 Oct 15;15(10):e0240848. doi: 10.1371/journal.pone.0240848. eCollection 2020. PLoS One. 2020. PMID: 33057384 Free PMC article.
-
Induction of ectopic taste buds by SHH reveals the competency and plasticity of adult lingual epithelium.Development. 2014 Aug;141(15):2993-3002. doi: 10.1242/dev.107631. Epub 2014 Jul 3. Development. 2014. PMID: 24993944 Free PMC article.
-
Early taste buds are from Shh+ epithelial cells of tongue primordium in distinction from mature taste bud cells which arise from surrounding tissue compartments.Biochem Biophys Res Commun. 2019 Jul 12;515(1):149-155. doi: 10.1016/j.bbrc.2019.05.132. Epub 2019 May 24. Biochem Biophys Res Commun. 2019. PMID: 31133375 Free PMC article.
-
Tongue and Taste Organ Biology and Function: Homeostasis Maintained by Hedgehog Signaling.Annu Rev Physiol. 2017 Feb 10;79:335-356. doi: 10.1146/annurev-physiol-022516-034202. Annu Rev Physiol. 2017. PMID: 28192057 Free PMC article. Review.
-
Progress and renewal in gustation: new insights into taste bud development.Development. 2015 Nov 1;142(21):3620-9. doi: 10.1242/dev.120394. Development. 2015. PMID: 26534983 Free PMC article. Review.
Cited by
-
Organoid in dentistry: Models for oral biology and disease.J Dent Sci. 2025 Jul;20(3):1816-1823. doi: 10.1016/j.jds.2025.05.002. Epub 2025 May 15. J Dent Sci. 2025. PMID: 40654442 Free PMC article. Review.
-
Environmental and molecular control of tissue-specific ionocyte differentiation in zebrafish.Development. 2024 Oct 15;151(20):dev202809. doi: 10.1242/dev.202809. Epub 2024 Oct 22. Development. 2024. PMID: 39324331 Free PMC article.
-
Reassessing the genetic lineage tracing of lingual Lgr5+ and Lgr6+ cells in vivo.Anim Cells Syst (Seoul). 2024 Jul 21;28(1):353-366. doi: 10.1080/19768354.2024.2381578. eCollection 2024. Anim Cells Syst (Seoul). 2024. PMID: 39040684 Free PMC article.
-
Organoid models for chemosensing cell studies.Am J Physiol Cell Physiol. 2025 Jul 1;329(1):C136-C144. doi: 10.1152/ajpcell.00047.2025. Epub 2025 Jun 6. Am J Physiol Cell Physiol. 2025. PMID: 40478437 Free PMC article. Review.
-
Environmental and molecular control of tissue-specific ionocyte differentiation in zebrafish.bioRxiv [Preprint]. 2024 Jan 13:2024.01.12.575421. doi: 10.1101/2024.01.12.575421. bioRxiv. 2024. Update in: Development. 2024 Oct 15;151(20):dev202809. doi: 10.1242/dev.202809. PMID: 38260427 Free PMC article. Updated. Preprint.
References
-
- An, W. F., Germain, A. R., Bishop, J. A., Nag, P. P., Metkar, S., Ketterman, J., Walk, M., Weiwer, M., Liu, X., Patnaik, D.et al. (2010). Discovery of potent and highly selective inhibitors of GSK3b. In Probe Reports from the NIH Molecular Libraries Program, pp. 1-115. www.ncbi.nlm.nih.gov/books/NBK133436
-
- Arnold, K., Sarkar, A., Yram, M. A., Polo, J. M., Bronson, R., Sengupta, S., Seandel, M., Geijsen, N. and Hochedlinger, K. (2011). Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9, 317-329. 10.1016/j.stem.2011.09.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

