Association of Immune-Related Adverse Events With Efficacy of Atezolizumab in Patients With Non-Small Cell Lung Cancer: Pooled Analyses of the Phase 3 IMpower130, IMpower132, and IMpower150 Randomized Clinical Trials
- PMID: 36795388
- PMCID: PMC9936386
- DOI: 10.1001/jamaoncol.2022.7711
Association of Immune-Related Adverse Events With Efficacy of Atezolizumab in Patients With Non-Small Cell Lung Cancer: Pooled Analyses of the Phase 3 IMpower130, IMpower132, and IMpower150 Randomized Clinical Trials
Erratum in
-
Errors in Affiliation, Figure 3, and Supplement 4.JAMA Oncol. 2023 Apr 1;9(4):574. doi: 10.1001/jamaoncol.2023.0822. JAMA Oncol. 2023. PMID: 37078994 Free PMC article. No abstract available.
Abstract
Importance: Immune-related adverse events (irAEs) arising from immune checkpoint inhibitor (ICI) cancer therapy may potentially predict improved outcomes.
Objective: To evaluate the association between irAEs and atezolizumab efficacy in patients with advanced non-small cell lung cancer (NSCLC) using pooled data from 3 phase 3 ICI studies.
Design, setting, and participants: IMpower130, IMpower132, and IMpower150 were phase 3, multicenter, open-label, randomized clinical trials to evaluate the efficacy and safety of chemoimmunotherapy combinations involving atezolizumab. Participants were chemotherapy-naive adults with stage IV nonsquamous NSCLC. These post hoc analyses were conducted during February 2022.
Interventions: Eligible patients were randomly assigned 2:1 to receive atezolizumab with carboplatin plus nab-paclitaxel, or chemotherapy alone (IMpower130); 1:1 to receive atezolizumab with carboplatin or cisplatin plus pemetrexed, or chemotherapy alone (IMpower132); and 1:1:1 to receive atezolizumab plus bevacizumab plus carboplatin and paclitaxel, atezolizumab plus carboplatin and paclitaxel, or bevacizumab plus carboplatin and paclitaxel (IMpower150).
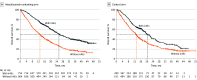
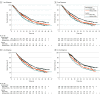
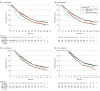
Main outcomes and measures: Pooled data from IMpower130 (cutoff: March 15, 2018), IMpower132 (cutoff: May 22, 2018), and IMpower150 (cutoff: September 13, 2019) were analyzed by treatment (atezolizumab-containing vs control), irAE status (with vs without), and highest irAE grade (1-2 vs 3-5). To account for immortal bias, a time-dependent Cox model and landmark analyses of irAE occurrence at 1, 3, 6, and 12 months from baseline were used to estimate the hazard ratio (HR) of overall survival (OS).
Results: Of 2503 randomized patients, 1577 were in the atezolizumab-containing arm and 926 were in the control arm. The mean (SD) age of patients was 63.1 (9.4) years and 63.0 (9.3) years, and 950 (60.2%) and 569 (61.4%) were male, respectively, in the atezolizumab arm and the control arm. Baseline characteristics were generally balanced between patients with irAEs (atezolizumab, n = 753; control, n = 289) and without (atezolizumab, n = 824; control, n = 637). In the atezolizumab arm, OS HRs (95% CI) in patients with grade 1 to 2 irAEs and grade 3 to 5 irAEs (each vs those without irAEs) in the 1-, 3-, 6-, and 12-month subgroups were 0.78 (0.65-0.94) and 1.25 (0.90-1.72), 0.74 (0.63-0.87) and 1.23 (0.93-1.64), 0.77 (0.65-0.90) and 1.1 (0.81-1.42), and 0.72 (0.59-0.89) and 0.87 (0.61-1.25), respectively.
Conclusions and relevance: In this pooled analysis of 3 randomized clinical trials, longer OS was observed in patients with vs without mild to moderate irAEs in both arms and across landmarks. These findings further support the use of first-line atezolizumab-containing regimens for advanced nonsquamous NSCLC.
Trial registration: ClinicalTrials.gov Identifiers: NCT02367781, NCT02657434, and NCT02366143.
Conflict of interest statement
Figures
References
-
- Tang SQ, Tang LL, Mao YP, et al. The pattern of time to onset and resolution of immune-related adverse events caused by immune checkpoint inhibitors in cancer: a pooled analysis of 23 clinical trials and 8,436 patients. Cancer Res Treat. 2021;53(2):339-354. doi: 10.4143/crt.2020.790 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

