Effectiveness of bivalent mRNA booster vaccination against SARS-CoV-2 Omicron infection, the Netherlands, September to December 2022
- PMID: 36795500
- PMCID: PMC9936593
- DOI: 10.2807/1560-7917.ES.2023.28.7.2300087
Effectiveness of bivalent mRNA booster vaccination against SARS-CoV-2 Omicron infection, the Netherlands, September to December 2022
Abstract
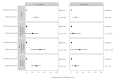
We used data of 32,542 prospective cohort study participants who previously received primary and one or two monovalent booster COVID-19 vaccinations. Between 26 September and 19 December 2022, relative effectiveness of bivalent original/Omicron BA.1 vaccination against self-reported Omicron SARS-CoV-2 infection was 31% in 18-59-year-olds and 14% in 60-85-year-olds. Protection of Omicron infection was higher than of bivalent vaccination without prior infection. Although bivalent booster vaccination increases protection against COVID-19 hospitalisations, we found limited added benefit in preventing SARS-CoV-2 infection.
Keywords: COVID-19; SARS-CoV-2; vaccine effectiveness.
Conflict of interest statement
Figures


References
-
- World Health Organization (WHO). One year since the emergence of COVID-19 virus variant Omicron. Geneva: WHO; 2022. Available from: https://www.who.int/news-room/feature-stories/detail/one-year-since-the-...
-
- European Medicines Agency (EMA). First adapted COVID-19 booster vaccines recommended for approval in the EU. EMA; 2022. Available from: https://www.ema.europa.eu/en/news/first-adapted-covid-19-booster-vaccine...
-
- Rijksinstituut voor Volksgezondheid en Milieu (RIVM). Varianten van het coronavirus SARS-CoV-2.[Variants of the coronavirus SARS-CoV-2]. Bilthoven: RIVM; 2023. Dutch. Available from: https://www.rivm.nl/coronavirus-covid-19/virus/varianten
-
- Huiberts A, Kooijman M, de Melker H, Hahne S, Grobbee D, Hoeve C, et al. Design and baseline description of an observational population-based cohort study on COVID-19 vaccine effectiveness in the Netherlands - The VAccine Study COvid-19 (VASCO) [PREPRINT]. Research Square. 2022. 10.21203/rs.3.rs-1645696/v1 - DOI
-
- Huiberts AJ, de Gier B, Hoeve CE, de Melker HE, Hahné SJM, Hartog Gd, et al. Vaccine effectiveness of primary and booster COVID-19 vaccinations against SARS-CoV-2 infection in the Netherlands from 12 July 2021 to 6 June 2022: a prospective cohort study [PREPRINT]. medRxiv. 2023:2023.01.09.23284335. 10.1101/2023.01.09.23284335 10.1101/2023.01.09.23284335 - DOI - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
