Cone photoreceptors transfer damaged mitochondria to Müller glia
- PMID: 36795565
- PMCID: PMC10425575
- DOI: 10.1016/j.celrep.2023.112115
Cone photoreceptors transfer damaged mitochondria to Müller glia
Abstract
Mitochondria are vital organelles that require sophisticated homeostatic mechanisms for maintenance. Intercellular transfer of damaged mitochondria is a recently identified strategy broadly used to improve cellular health and viability. Here, we investigate mitochondrial homeostasis in the vertebrate cone photoreceptor, the specialized neuron that initiates our daytime and color vision. We find a generalizable response to mitochondrial stress that leads to loss of cristae, displacement of damaged mitochondria from their normal cellular location, initiation of degradation, and transfer to Müller glia cells, a key non-neuronal support cell in the retina. Our findings show transmitophagy from cones to Müller glia as a response to mitochondrial damage. Intercellular transfer of damaged mitochondria represents an outsourcing mechanism that photoreceptors use to support their specialized function.
Keywords: CP: Neuroscience; Müller glia; mitochondria; mitophagy; photoreceptor; retina; zebrafish.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
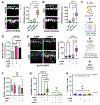
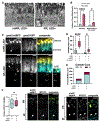
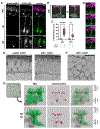
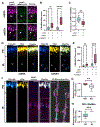
References
-
- Rosina M, Ceci V, Turchi R, Chuan L, Borcherding N, Sciarretta F, Sánchez-Díaz M, Tortolici F, Karlinsey K, Chiurchiù V, et al. (2022). Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metabol. 34, 533–548.e12. 10.1016/j.cmet.2022.02.016. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

