Induction of flight via midbrain projections to the cuneiform nucleus
- PMID: 36795666
- PMCID: PMC9934373
- DOI: 10.1371/journal.pone.0281464
Induction of flight via midbrain projections to the cuneiform nucleus
Abstract
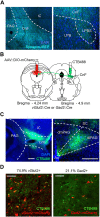
The dorsal periaqueductal gray is a midbrain structure implicated in the control of defensive behaviors and the processing of painful stimuli. Electrical stimulation or optogenetic activation of excitatory neurons in dorsal periaqueductal gray results in freezing or flight behavior at low and high intensity, respectively. However, the output structures that mediate these defensive behaviors remain unconfirmed. Here we carried out a targeted classification of neuron types in dorsal periaqueductal gray using multiplex in situ sequencing and then applied cell-type and projection-specific optogenetic stimulation to identify projections from dorsal periaqueductal grey to the cuneiform nucleus that promoted goal-directed flight behavior. These data confirmed that descending outputs from dorsal periaqueductal gray serve as a trigger for directed escape behavior.
Copyright: © 2023 Tsang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Neural segregation of Fos-protein distribution in the brain following freezing and escape behaviors induced by injections of either glutamate or NMDA into the dorsal periaqueductal gray of rats.Brain Res. 2005 Jan 21;1031(2):151-63. doi: 10.1016/j.brainres.2004.10.044. Brain Res. 2005. PMID: 15649440
-
Fos-like immunoreactive neurons following electrical stimulation of the dorsal periaqueductal gray at freezing and escape thresholds.Brain Res Bull. 2003 Dec 30;62(3):179-89. doi: 10.1016/j.brainresbull.2003.09.010. Brain Res Bull. 2003. PMID: 14698351
-
Inhibitory and excitatory projections from the dorsal raphe nucleus to neurons in the dorsolateral periaqueductal gray matter in slices of midbrain maintained in vitro.Neuroscience. 1994 Sep;62(1):177-87. doi: 10.1016/0306-4522(94)90323-9. Neuroscience. 1994. PMID: 7816199
-
Midbrain control of breathing and blood pressure: The role of periaqueductal gray matter and mesencephalic collicular neuronal microcircuit oscillators.Eur J Neurosci. 2020 Oct;52(8):3879-3902. doi: 10.1111/ejn.14727. Epub 2020 May 1. Eur J Neurosci. 2020. Retraction in: Eur J Neurosci. 2021 Oct;54(7):6685. doi: 10.1111/ejn.15484. PMID: 32227408 Retracted. Review.
-
The role of the periaqueductal gray in escape behavior.Curr Opin Neurobiol. 2020 Feb;60:115-121. doi: 10.1016/j.conb.2019.11.014. Epub 2019 Dec 19. Curr Opin Neurobiol. 2020. PMID: 31864105 Review.
Cited by
-
Tonically active GABAergic neurons in the dorsal periaqueductal gray control instinctive escape in mice.Curr Biol. 2024 Jul 8;34(13):3031-3039.e7. doi: 10.1016/j.cub.2024.05.068. Epub 2024 Jun 26. Curr Biol. 2024. PMID: 38936364 Free PMC article.
-
Central stress pathways in the development of cardiovascular disease.Clin Auton Res. 2024 Feb;34(1):99-116. doi: 10.1007/s10286-023-01008-x. Epub 2023 Dec 17. Clin Auton Res. 2024. PMID: 38104300 Review.
-
Inhibitory medial zona incerta pathway drives exploratory behavior by inhibiting glutamatergic cuneiform neurons.Nat Commun. 2024 Feb 7;15(1):1160. doi: 10.1038/s41467-024-45288-x. Nat Commun. 2024. PMID: 38326327 Free PMC article.
-
Brn3b regulates the formation of fear-related midbrain circuits and defensive responses to visual threat.PLoS Biol. 2023 Nov 20;21(11):e3002386. doi: 10.1371/journal.pbio.3002386. eCollection 2023 Nov. PLoS Biol. 2023. PMID: 37983249 Free PMC article.
-
Periaqueductal gray activates antipredatory neural responses in the amygdala of foraging rats.Elife. 2024 Aug 12;12:RP88733. doi: 10.7554/eLife.88733. Elife. 2024. PMID: 39133827 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

