HIV-1 prehairpin intermediate inhibitors show efficacy independent of neutralization tier
- PMID: 36795752
- PMCID: PMC9974412
- DOI: 10.1073/pnas.2215792120
HIV-1 prehairpin intermediate inhibitors show efficacy independent of neutralization tier
Abstract
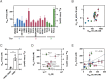
HIV-1 strains are categorized into one of three neutralization tiers based on the relative ease by which they are neutralized by plasma from HIV-1-infected donors not on antiretroviral therapy; tier-1 strains are particularly sensitive to neutralization while tier-2 and tier-3 strains are increasingly difficult to neutralize. Most broadly neutralizing antibodies (bnAbs) previously described target the native prefusion conformation of HIV-1 Envelope (Env), but the relevance of the tiered categories for inhibitors targeting another Env conformation, the prehairpin intermediate, is not well understood. Here, we show that two inhibitors targeting distinct highly conserved regions of the prehairpin intermediate have strikingly consistent neutralization potencies (within ~100-fold for a given inhibitor) against strains in all three neutralization tiers of HIV-1; in contrast, best-in-class bnAbs targeting diverse Env epitopes vary by more than 10,000-fold in potency against these strains. Our results indicate that antisera-based HIV-1 neutralization tiers are not relevant for inhibitors targeting the prehairpin intermediate and highlight the potential for therapies and vaccine efforts targeting this conformation.
Keywords: 5-Helix; HIV-1; prehairpin intermediate; tier-2 virus; tier-3 virus.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

