High PrEP uptake and objective longitudinal adherence among HIV-exposed women with personal or partner plans for pregnancy in rural Uganda: A cohort study
- PMID: 36795763
- PMCID: PMC9983833
- DOI: 10.1371/journal.pmed.1004088
High PrEP uptake and objective longitudinal adherence among HIV-exposed women with personal or partner plans for pregnancy in rural Uganda: A cohort study
Abstract
Background: In Uganda, fertility rates and adult HIV prevalence are high, and many women conceive with partners living with HIV. Pre-exposure prophylaxis (PrEP) reduces HIV acquisition for women and, therefore, infants. We developed the Healthy Families-PrEP intervention to support PrEP use as part of HIV prevention during periconception and pregnancy periods. We conducted a longitudinal cohort study to evaluate oral PrEP use among women participating in the intervention.
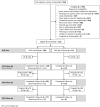
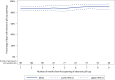
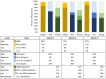
Methods and findings: We enrolled HIV-negative women with plans for pregnancy with a partner living, or thought to be living, with HIV (2017 to 2020) to evaluate PrEP use among women participating in the Healthy Families-PrEP intervention. Quarterly study visits through 9 months included HIV and pregnancy testing and HIV prevention counseling. PrEP was provided in electronic pillboxes, providing the primary adherence measure ("high" adherence when pillbox was opened ≥80% of days). Enrollment questionnaires assessed factors associated with PrEP use. Plasma tenofovir (TFV) and intraerythrocytic TFV-diphosphate (TFV-DP) concentrations were determined quarterly for women who acquired HIV and a randomly selected subset of those who did not; concentrations TFV ≥40 ng/mL and TFV-DP ≥600 fmol/punch were categorized as "high." Women who became pregnant were initially exited from the cohort by design; from March 2019, women with incident pregnancy remained in the study with quarterly follow-up until pregnancy outcome. Primary outcomes included (1) PrEP uptake (proportion who initiated PrEP); and (2) PrEP adherence (proportion of days with pillbox openings during the first 3 months following PrEP initiation). We used univariable and multivariable-adjusted linear regression to evaluate baseline predictors selected based on our conceptual framework of mean adherence over 3 months. We also assessed mean monthly adherence over 9 months of follow-up and during pregnancy. We enrolled 131 women with mean age 28.7 years (95% CI: 27.8 to 29.5). Ninety-seven (74%) reported a partner with HIV and 79 (60%) reported condomless sex. Most women (N = 118; 90%) initiated PrEP. Mean electronic adherence during the 3 months following initiation was 87% (95% CI: 83%, 90%). No covariates were associated with 3-month pill-taking behavior. Concentrations of plasma TFV and TFV-DP were high among 66% and 47%, 56% and 41%, and 45% and 45% at months 3, 6, and 9, respectively. We observed 53 pregnancies among 131 women (1-year cumulative incidence 53% [95% CI: 43%, 62%]) and 1 HIV-seroconversion in a non-pregnant woman. Mean pillcap adherence for PrEP users with pregnancy follow-up (N = 17) was 98% (95% CI: 97%, 99%). Study design limitations include lack of a control group.
Conclusions: Women in Uganda with PrEP indications and planning for pregnancy chose to use PrEP. By electronic pillcap, most were able to sustain high adherence to daily oral PrEP prior to and during pregnancy. Differences in adherence measures highlight challenges with adherence assessment; serial measures of TFV-DP in whole blood suggest 41% to 47% of women took sufficient periconception PrEP to prevent HIV. These data suggest that women planning for and with pregnancy should be prioritized for PrEP implementation, particularly in settings with high fertility rates and generalized HIV epidemics. Future iterations of this work should compare the outcomes to current standard of care.
Trial registration: ClinicalTrials.gov Identifier: NCT03832530 https://clinicaltrials.gov/ct2/show/NCT03832530?term=lynn+matthews&cond=hiv&cntry=UG&draw=2&rank=1.
Copyright: © 2023 Matthews et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
LTM has received clinical research funding from Gilead Sciences. CWH has received clinical research funding from Gilead Sciences and Merck and, holds patents related to HIV prevention products licensed by Johns Hopkins to Prionde Biopharma, LLC, which he founded. PLA has received consultant fees from Merck, ViiV, and Gilead and research support from Gilead. JEH has received consultant fees from Merck and Natera. KEH is an employee and a shareholder of NoviSci/Target RWE, which has received fees from Janssen Research & Development (Janssen R&D) and Amgen Inc. outside the submitted work.
Figures


References
-
- The Republic of Uganda, Ministry of Health. The 2019 HIV Epidemiological Surveillance Report for Uganda. 2020.
-
- The World Bank. Fertility rate, total (births per woman)–Uganda. 2020 [cited 2022 Nov 1]. Available from: https://data.worldbank.org/indicator/SP.DYN.TFRT.IN?locations=UG.
-
- Beyeza-Kashesya J, Ekstrom AM, Kaharuza F, Mirembe F, Neema S, Kulane A. My partner wants a child: a cross-sectional study of the determinants of the desire for children among mutually disclosed sero-discordant couples receiving care in Uganda. BMC Public Health. 2010;10:247. doi: 1471-2458-10-247 [pii]. doi: 10.1186/1471-2458-10-247 ; PubMed Central PMCID: PMC2877675. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

