Rucaparib or Physician's Choice in Metastatic Prostate Cancer
- PMID: 36795891
- PMCID: PMC10064172
- DOI: 10.1056/NEJMoa2214676
Rucaparib or Physician's Choice in Metastatic Prostate Cancer
Abstract
Background: In a phase 2 study, rucaparib, an inhibitor of poly(ADP-ribose) polymerase (PARP), showed a high level of activity in patients who had metastatic, castration-resistant prostate cancer associated with a deleterious BRCA alteration. Data are needed to confirm and expand on the findings of the phase 2 study.
Methods: In this randomized, controlled, phase 3 trial, we enrolled patients who had metastatic, castration-resistant prostate cancer with a BRCA1, BRCA2, or ATM alteration and who had disease progression after treatment with a second-generation androgen-receptor pathway inhibitor (ARPI). We randomly assigned the patients in a 2:1 ratio to receive oral rucaparib (600 mg twice daily) or a physician's choice control (docetaxel or a second-generation ARPI [abiraterone acetate or enzalutamide]). The primary outcome was the median duration of imaging-based progression-free survival according to independent review.
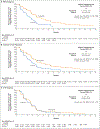
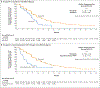
Results: Of the 4855 patients who had undergone prescreening or screening, 270 were assigned to receive rucaparib and 135 to receive a control medication (intention-to-treat population); in the two groups, 201 patients and 101 patients, respectively, had a BRCA alteration. At 62 months, the duration of imaging-based progression-free survival was significantly longer in the rucaparib group than in the control group, both in the BRCA subgroup (median, 11.2 months and 6.4 months, respectively; hazard ratio, 0.50; 95% confidence interval [CI], 0.36 to 0.69) and in the intention-to-treat group (median, 10.2 months and 6.4 months, respectively; hazard ratio, 0.61; 95% CI, 0.47 to 0.80; P<0.001 for both comparisons). In an exploratory analysis in the ATM subgroup, the median duration of imaging-based progression-free survival was 8.1 months in the rucaparib group and 6.8 months in the control group (hazard ratio, 0.95; 95% CI, 0.59 to 1.52). The most frequent adverse events with rucaparib were fatigue and nausea.
Conclusions: The duration of imaging-based progression-free survival was significantly longer with rucaparib than with a control medication among patients who had metastatic, castration-resistant prostate cancer with a BRCA alteration. (Funded by Clovis Oncology; TRITON3 ClinicalTrials.gov number, NCT02975934.).
Copyright © 2023 Massachusetts Medical Society.
Figures

Comment in
-
Rucaparib improves PFS in patients with BRCA1/2-altered mCRPC.Nat Rev Urol. 2023 Apr;20(4):199. doi: 10.1038/s41585-023-00760-z. Nat Rev Urol. 2023. PMID: 36928618 No abstract available.
-
Urologic Oncology: Prostate Cancer.J Urol. 2023 Sep;210(3):565-567. doi: 10.1097/JU.0000000000003587. Epub 2023 Jun 19. J Urol. 2023. PMID: 37334535 No abstract available.
-
Rucaparib for metastatic castration-resistant prostate cancer: did TRITON3 deliver a trifecta?Transl Cancer Res. 2023 Oct 31;12(10):2448-2453. doi: 10.21037/tcr-23-1279. Epub 2023 Oct 3. Transl Cancer Res. 2023. PMID: 37969378 Free PMC article. No abstract available.
-
The growing role of PARP inhibitors in the treatment of metastatic castration-resistant prostate cancer.Transl Cancer Res. 2023 Dec 31;12(12):3233-3240. doi: 10.21037/tcr-23-1515. Epub 2023 Nov 13. Transl Cancer Res. 2023. PMID: 38192981 Free PMC article. No abstract available.
-
How far does a new horizon extend for rucaparib in metastatic prostate cancer?Transl Cancer Res. 2024 Jan 31;13(1):11-14. doi: 10.21037/tcr-23-1563. Epub 2024 Jan 12. Transl Cancer Res. 2024. PMID: 38410224 Free PMC article. No abstract available.
References
-
- Gillessen S, Armstrong A, Attard G, et al. Management of patients with advanced prostate cancer: report from the Advanced Prostate Cancer Consensus Conference 2021. Eur Urol 2022;82:115–41. - PubMed
-
- Vogl UM, Beer TM, Davis ID, et al. Lack of consensus identifies important areas for future clinical research: Advanced Prostate Cancer Consensus Conference (APCCC) 2019 findings. Eur J Cancer 2022;160:24–60. - PubMed
-
- Sayegh N, Swami U, Agarwal N. Recent advances in the management of metastatic prostate cancer. JCO Oncol Pract 2022;18:45–55. - PubMed
-
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917–21. - PubMed
-
- Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434:913–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
