Dual Photochemistry of Benzimidazole
- PMID: 36795993
- PMCID: PMC9990075
- DOI: 10.1021/acs.joc.2c02560
Dual Photochemistry of Benzimidazole
Abstract
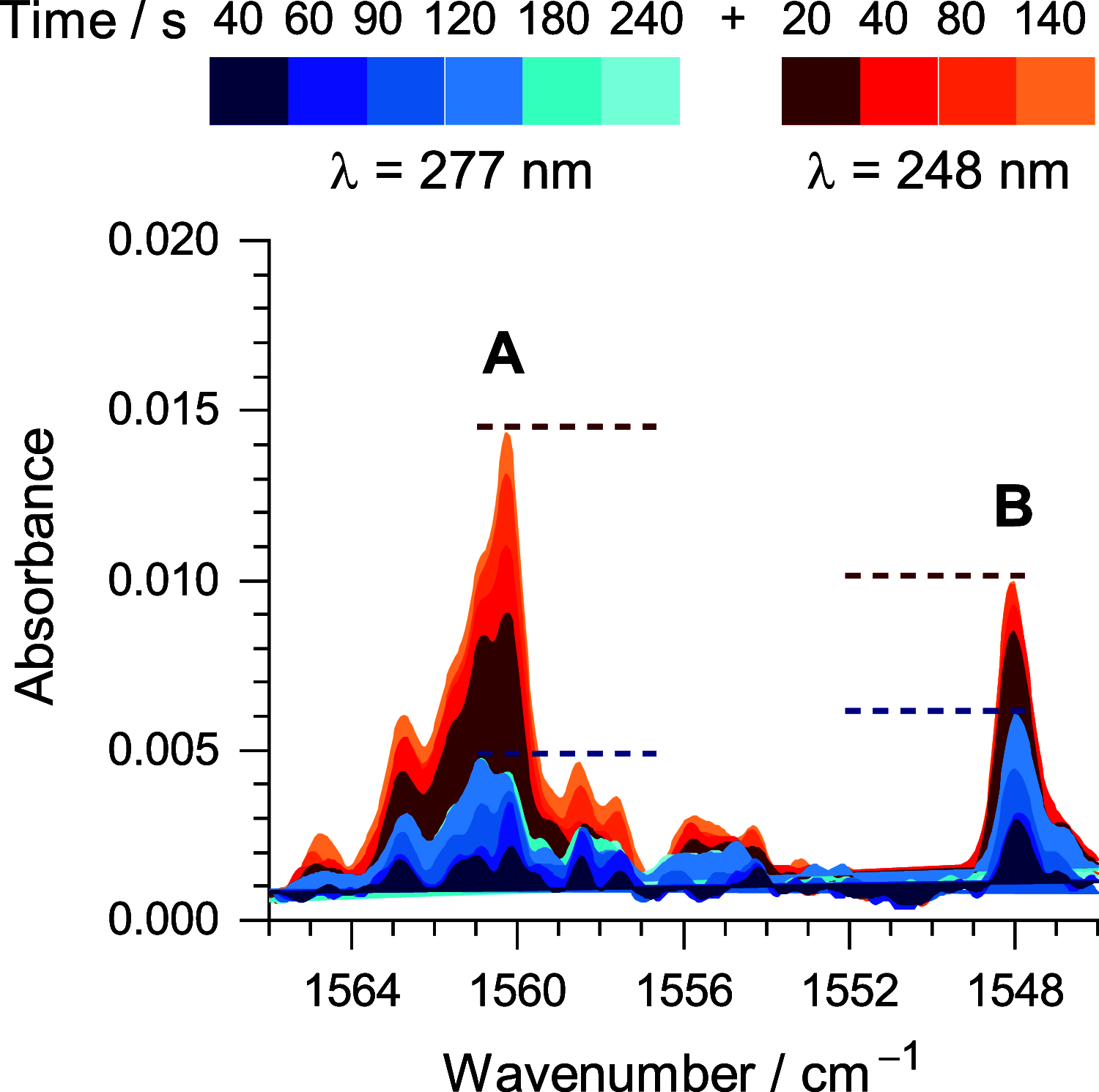
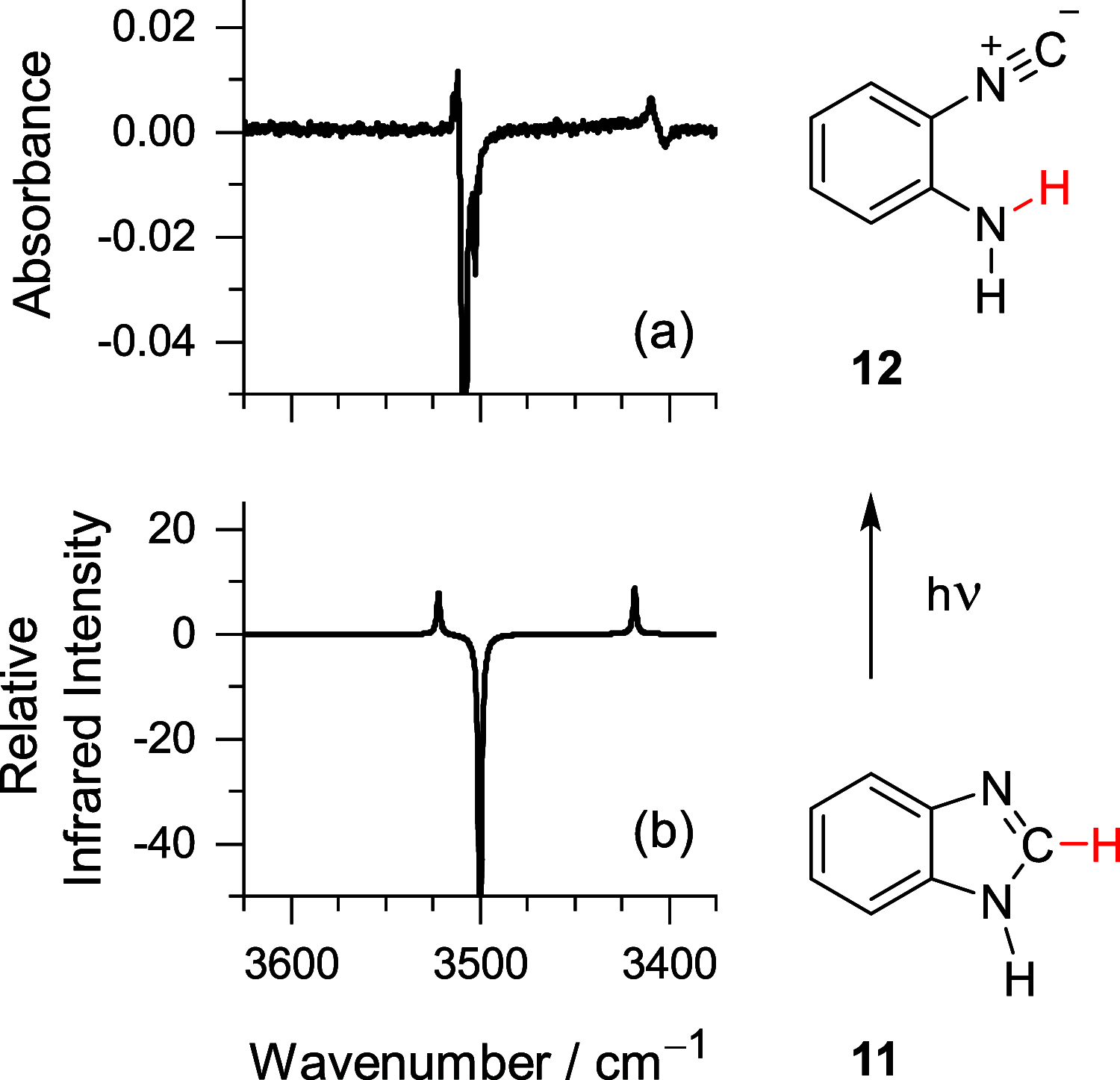
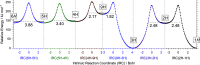
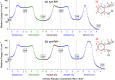
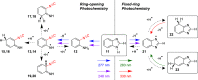
Monomers of benzimidazole trapped in an argon matrix at 15 K were characterized by vibrational spectroscopy and identified as 1H-tautomers exclusively. The photochemistry of matrix-isolated 1H-benzimidazole was induced by excitations with a frequency-tunable narrowband UV light and followed spectroscopically. Hitherto unobserved photoproducts were identified as 4H- and 6H-tautomers. Simultaneously, a family of photoproducts bearing the isocyano moiety was identified. Thereby, the photochemistry of benzimidazole was hypothesized to follow two reaction pathways: the fixed-ring and the ring-opening isomerizations. The former reaction channel results in the cleavage of the NH bond and formation of a benzimidazolyl radical and an H-atom. The latter reaction channel involves the cleavage of the five-membered ring and concomitant shift of the H-atom from the CH bond of the imidazole moiety to the neighboring NH group, leading to 2-isocyanoaniline and subsequently to the isocyanoanilinyl radical. The mechanistic analysis of the observed photochemistry suggests that detached H-atoms, in both cases, recombine with the benzimidazolyl or isocyanoanilinyl radicals, predominantly at the positions with the largest spin density (revealed using the natural bond analysis computations). The photochemistry of benzimidazole therefore occupies an intermediate position between the earlier studied prototype cases of indole and benzoxazole, which exhibit exclusively the fixed-ring and the ring-opening photochemistries, respectively.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Woolley D. W. Some biological effects produced by benzimidazole and their reversal by purines. J. Biol. Chem. 1944, 152, 225–232. 10.1016/s0021-9258(18)72045-0. - DOI
-
- Fischer O.; Rigaud M. Ueber Benzimidazole. Ber. Dtsch. Chem. Ges. 1902, 35, 1258–1265. 10.1002/cber.19020350209. - DOI
-
- Fischer O.; Limmer F. Über Benzimidazole und deren Aufspaltung. J. Prakt. Chem. 1906, 74, 57–73. 10.1002/prac.19060740105. - DOI
-
- Fischer O. Über die Tautomeriefrage bei den Benzimidazolen. J. Prakt. Chem. 1907, 75, 88–93. 10.1002/prac.19070750104. - DOI
-
- Saxena A.; Hegde V.; Mutalikdesai S.; Maste M. Versatility of Benzimidazole and Its Derivatives; an Insight. Int. J. Pharm. Sci. Res. 2020, 11, 4152–4173. 10.13040/IJPSR.0975-8232.11(9).4152-73. - DOI
LinkOut - more resources
Full Text Sources

