Chitosan-Modified Polyethyleneimine Nanoparticles for Enhancing the Carboxylation Reaction and Plants' CO2 Uptake
- PMID: 36796108
- PMCID: PMC9979637
- DOI: 10.1021/acsnano.2c09255
Chitosan-Modified Polyethyleneimine Nanoparticles for Enhancing the Carboxylation Reaction and Plants' CO2 Uptake
Abstract
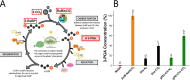
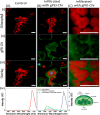
Increasing plants' photosynthetic efficiency is a major challenge that must be addressed in order to cover the food demands of the growing population in the changing climate. Photosynthesis is greatly limited at the initial carboxylation reaction, where CO2 is converted to the organic acid 3-PGA, catalyzed by the RuBisCO enzyme. RuBisCO has poor affinity for CO2, but also the CO2 concentration at the RuBisCO site is limited by the diffusion of atmospheric CO2 through the various leaf compartments to the reaction site. Beyond genetic engineering, nanotechnology can offer a materials-based approach for enhancing photosynthesis, and yet, it has mostly been explored for the light-dependent reactions. In this work, we developed polyethyleneimine-based nanoparticles for enhancing the carboxylation reaction. We demonstrate that the nanoparticles can capture CO2 in the form of bicarbonate and increase the CO2 that reacts with the RuBisCO enzyme, enhancing the 3-PGA production in in vitro assays by 20%. The nanoparticles can be introduced to the plant via leaf infiltration and, because of the functionalization with chitosan oligomers, they do not induce any toxic effect to the plant. In the leaves, the nanoparticles localize in the apoplastic space but also spontaneously reach the chloroplasts where photosynthetic activity takes place. Their CO2 loading-dependent fluorescence verifies that, in vivo, they maintain their ability to capture CO2 and can be therefore reloaded with atmospheric CO2 while in planta. Our results contribute to the development of a nanomaterials-based CO2-concentrating mechanism in plants that can potentially increase photosynthetic efficiency and overall plants' CO2 storage.
Keywords: CO2 capture; chitosan; nanoparticles; photosynthesis; polyethyleneimine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Martin W., Scheibe R., Schnarrenberger C.. The Calvin Cycle and Its Regulation. In Photosynthesis. Advances in Photosynthesis and Respiration; Leegood R. C., Sharkey T. D., von Caemmerer S., Eds.; Springer: Dordrecht, 2000; Vol. 9, pp 9–51.
-
- Sharkey T. D. Estimating the Rate of Photorespiration in Leaves. Physiol. Plant. 1988, 73, 147–152. 10.1111/j.1399-3054.1988.tb09205.x. - DOI
-
- Borovsky D.; Smith E. E.; Whelan W. J. Purification and Properties of Potato 1,4-Alpha-d-Glucan: 1,4-Alpha-d-Glucan 6-Alpha-(1,4-Alpha-Glucano)-Transferase. Evidence against a Dual Catalytic Function in Amylose-Branching Enzyme. Eur. J. Biochem. 1975, 59, 615–625. 10.1111/j.1432-1033.1975.tb02490.x. - DOI - PubMed

