Selective emergence of antibody-secreting cells in the multiple sclerosis brain
- PMID: 36796230
- PMCID: PMC9958261
- DOI: 10.1016/j.ebiom.2023.104465
Selective emergence of antibody-secreting cells in the multiple sclerosis brain
Abstract
Background: Although distinct brain-homing B cells have been identified in multiple sclerosis (MS), it is unknown how these further evolve to contribute to local pathology. We explored B-cell maturation in the central nervous system (CNS) of MS patients and determined their association with immunoglobulin (Ig) production, T-cell presence, and lesion formation.
Methods: Ex vivo flow cytometry was performed on post-mortem blood, cerebrospinal fluid (CSF), meninges and white matter from 28 MS and 10 control brain donors to characterize B cells and antibody-secreting cells (ASCs). MS brain tissue sections were analysed with immunostainings and microarrays. IgG index and CSF oligoclonal bands were measured with nephelometry, isoelectric focusing, and immunoblotting. Blood-derived B cells were cocultured under T follicular helper-like conditions to evaluate their ASC-differentiating capacity in vitro.
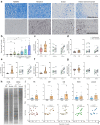
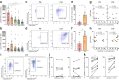
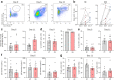
Findings: ASC versus B-cell ratios were increased in post-mortem CNS compartments of MS but not control donors. Local presence of ASCs associated with a mature CD45low phenotype, focal MS lesional activity, lesional Ig gene expression, and CSF IgG levels as well as clonality. In vitro B-cell maturation into ASCs did not differ between MS and control donors. Notably, lesional CD4+ memory T cells positively correlated with ASC presence, reflected by local interplay with T cells.
Interpretation: These findings provide evidence that local B cells at least in late-stage MS preferentially mature into ASCs, which are largely responsible for intrathecal and local Ig production. This is especially seen in active MS white matter lesions and likely depends on the interaction with CD4+ memory T cells.
Funding: Stichting MS Research (19-1057 MS; 20-490f MS), National MS Fonds (OZ2018-003).
Keywords: B-cell maturation; CXCR3; Central nervous system; Immunoglobulins; T cells; White matter lesions.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests M.M.v.L. received research support from EMD Serono, Merck, GSK and Idorsia Pharmaceutical Ltd. J.S. received lecture and/or consultancy fees from Biogen, Merck, Novartis and Sanofi-Genzyme. The remaining authors declare no competing interests.
Figures


References
-
- Meffre E., Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20:632–638. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

