Hypothalamic neurons that mirror aggression
- PMID: 36796363
- PMCID: PMC10081867
- DOI: 10.1016/j.cell.2023.01.022
Hypothalamic neurons that mirror aggression
Erratum in
-
Hypothalamic neurons that mirror aggression.Cell. 2024 Jul 11;187(14):3782. doi: 10.1016/j.cell.2024.05.046. Epub 2024 May 29. Cell. 2024. PMID: 38815581 Free PMC article. No abstract available.
Abstract
Social interactions require awareness and understanding of the behavior of others. Mirror neurons, cells representing an action by self and others, have been proposed to be integral to the cognitive substrates that enable such awareness and understanding. Mirror neurons of the primate neocortex represent skilled motor tasks, but it is unclear if they are critical for the actions they embody, enable social behaviors, or exist in non-cortical regions. We demonstrate that the activity of individual VMHvlPR neurons in the mouse hypothalamus represents aggression performed by self and others. We used a genetically encoded mirror-TRAP strategy to functionally interrogate these aggression-mirroring neurons. We find that their activity is essential for fighting and that forced activation of these cells triggers aggressive displays by mice, even toward their mirror image. Together, we have discovered a mirroring center in an evolutionarily ancient region that provides a subcortical cognitive substrate essential for a social behavior.
Keywords: FosTRAP; TRAP2; VMHvl; aggression; cognition; emotion; fiber photometry; miniscope; mirror neurons; social behavior; social cognition; tail rattle; ventromedial hypothalamus.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.L. is a member of the advisory board for Cell.
Figures


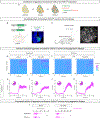

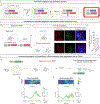
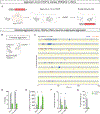

Comment in
-
'Mirror neurons' fire up during mouse battles.Nature. 2023 Feb;614(7949):605. doi: 10.1038/d41586-023-00418-1. Nature. 2023. PMID: 36792906 No abstract available.
-
To see is to experience: Aggression neurons light up when witnessing a fight.Cell. 2023 Mar 16;186(6):1099-1100. doi: 10.1016/j.cell.2023.01.025. Epub 2023 Mar 1. Cell. 2023. PMID: 36863338
References
-
- Swaab D (2003). The ventromedial nucleus (VMN; nucleus of Cajal). In The Human Hypothalamus: Basic and Clinical Aspects Part I: Nuclei of the Human Hypothalamus (Elsevier; ), pp. 239–242.
-
- Hess WR, and Akert K (1955). Experimental data on role of hypothalamus in mechanism of emotional behavior. AMA Arch Neurol Psychiatry 73, 127–129. - PubMed
-
- Kruk MR (1991). Ethology and pharmacology of hypothalamic aggression in the rat. Neurosci Biobehav Rev 15, 527–538. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

