Who Needs Epinephrine? Anaphylaxis, Autoinjectors, and Parachutes
- PMID: 36796511
- PMCID: PMC10259181
- DOI: 10.1016/j.jaip.2023.02.002
Who Needs Epinephrine? Anaphylaxis, Autoinjectors, and Parachutes
Abstract
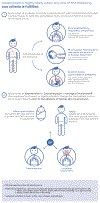
International guidelines stipulate that intramuscular (IM) epinephrine (adrenaline) is the first-line treatment for anaphylaxis, with an established good safety profile. The availability of epinephrine autoinjectors (EAI) has greatly facilitated the lay administration of IM epinephrine in community settings. However, key areas of uncertainty remain around epinephrine usage. These include variations in prescribing EAI, what symptoms should prompt epinephrine administration, whether emergency medical services (EMS) need to be contacted after administration, and whether epinephrine administered via EAI reduces mortality from anaphylaxis or improves quality of life measures. We provide a balanced commentary on these issues. There is increasing recognition that a poor response to epinephrine, particularly after 2 doses, is a useful marker of severity and the need for urgent escalation. It is likely that patients who respond to a single epinephrine dose do not require EMS activation or emergency department transfer, but data are needed to demonstrate the safety of this approach. Lastly, patients at risk of anaphylaxis must be counseled against over-reliance on EAI alone.
Keywords: Anaphylaxis; Autoinjector; Epinephrine; Outcomes; Prescribing.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL, et al. Joint Task Force on Practice Parameters Reviewers. Anaphylaxis—a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol 2020;145:1082–123. - PubMed
-
- Muraro A, Worm M, Alviani C, Cardona V, DunnGalvin A, Garvey LH, et al. European Academy of Allergy and Clinical Immunology, Food Allergy, Anaphylaxis Guidelines Group. EAACI guidelines: anaphylaxis (2021 update). Allergy 2022;77:357–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

