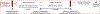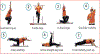Design and rationale of re-energize fontan: Randomized exercise intervention designed to maximize fitness in fontan patients
- PMID: 36796574
- PMCID: PMC10085861
- DOI: 10.1016/j.ahj.2023.02.006
Design and rationale of re-energize fontan: Randomized exercise intervention designed to maximize fitness in fontan patients
Abstract
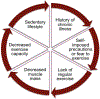
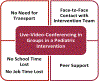
In this manuscript, we describe the design and rationale of a randomized controlled trial in pediatric Fontan patients to test the hypothesis that a live-video-supervised exercise (aerobic+resistance) intervention will improve cardiac and physical capacity; muscle mass, strength, and function; and endothelial function. Survival of children with single ventricles beyond the neonatal period has increased dramatically with the staged Fontan palliation. Yet, long-term morbidity remains high. By age 40, 50% of Fontan patients will have died or undergone heart transplantation. Factors that contribute to onset and progression of heart failure in Fontan patients remain incompletely understood. However, it is established that Fontan patients have poor exercise capacity which is associated with a greater risk of morbidity and mortality. Furthermore, decreased muscle mass, abnormal muscle function, and endothelial dysfunction in this patient population is known to contribute to disease progression. In adult patients with 2 ventricles and heart failure, reduced exercise capacity, muscle mass, and muscle strength are powerful predictors of poor outcomes, and exercise interventions can not only improve exercise capacity and muscle mass, but also reverse endothelial dysfunction. Despite these known benefits of exercise, pediatric Fontan patients do not exercise routinely due to their chronic condition, perceived restrictions to exercise, and parental overprotection. Limited exercise interventions in children with congenital heart disease have demonstrated that exercise is safe and effective; however, these studies have been conducted in small, heterogeneous groups, and most had few Fontan patients. Critically, adherence is a major limitation in pediatric exercise interventions delivered on-site, with adherence rates as low as 10%, due to distance from site, transportation difficulties, and missed school or workdays. To overcome these challenges, we utilize live-video conferencing to deliver the supervised exercise sessions. Our multidisciplinary team of experts will assess the effectiveness of a live-video-supervised exercise intervention, rigorously designed to maximize adherence, and improve key and novel measures of health in pediatric Fontan patients associated with poor long-term outcomes. Our ultimate goal is the translation of this model to clinical application as an "exercise prescription" to intervene early in pediatric Fontan patients and decrease long-term morbidity and mortality.
Trial registration: ClinicalTrials.gov NCT04195451.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures