Extracellular vesicles derived from Porphyromonas gingivalis induce trigeminal nerve-mediated cognitive impairment
- PMID: 36796586
- PMCID: PMC10703712
- DOI: 10.1016/j.jare.2023.02.006
Extracellular vesicles derived from Porphyromonas gingivalis induce trigeminal nerve-mediated cognitive impairment
Abstract
Introduction: Porphyromonas gingivalis (PG)-infected periodontitis is in close connection with the development of Alzheimer's disease (AD). PG-derived extracellular vesicles (pEVs) contain inflammation-inducing virulence factors, including gingipains (GPs) and lipopolysaccharide (LPS).
Objectives: To understand how PG could cause cognitive decline, we investigated the effects of PG and pEVs on the etiology of periodontitis and cognitive impairment in mice.
Methods: Cognitive behaviors were measured in the Y-maze and novel object recognition tasks. Biomarkers were measured using ELISA, qPCR, immunofluorescence assay, and pyrosequencing.
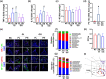
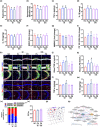
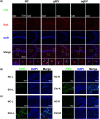
Results: pEVs contained neurotoxic GPs and inflammation-inducible fimbria protein and LPS. Gingivally exposed, but not orally gavaged, PG or pEVs caused periodontitis and induced memory impairment-like behaviors. Gingival exposure to PG or pEVs increased TNF-α expression in the periodontal and hippocampus tissues. They also increased hippocampal GP+Iba1+, LPS+Iba1+, and NF-κB+Iba1+ cell numbers. Gingivally exposed PG or pEVs decreased BDNF, claudin-5, and N-methyl-D-aspartate receptor expression and BDNF+NeuN+ cell number. Gingivally exposed fluorescein-5-isothiocyanate-labeled pEVs (F-pEVs) were detected in the trigeminal ganglia and hippocampus. However, right trigeminal neurectomy inhibited the translocation of gingivally injected F-EVs into the right trigeminal ganglia. Gingivally exposed PG or pEVs increased blood LPS and TNF-α levels. Furthermore, they caused colitis and gut dysbiosis.
Conclusion: Gingivally infected PG, particularly pEVs, may cause cognitive decline with periodontitis. PG products pEVs and LPS may be translocated into the brain through the trigeminal nerve and periodontal blood pathways, respectively, resulting in the cognitive decline, which may cause colitis and gut dysbiosis. Therefore, pEVs may be a remarkable risk factor for dementia.
Keywords: Extracellular vesicle; Memory impairment; Microbiota dysbiosis; Periodontitis; Porphyromonas gingivalis.
Copyright © 2023. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Peila R, Launer LJ. Inflammation and dementia: epidemiologic evidence. Acta Neurol Scand Suppl. 2006;185:102–6. doi:10.1111/j.1600-0404.2006.00693.x. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

