The Proteome of Large or Small Extracellular Vesicles in Pig Seminal Plasma Differs, Defining Sources and Biological Functions
- PMID: 36796643
- PMCID: PMC10017305
- DOI: 10.1016/j.mcpro.2023.100514
The Proteome of Large or Small Extracellular Vesicles in Pig Seminal Plasma Differs, Defining Sources and Biological Functions
Abstract
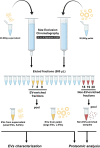
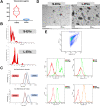
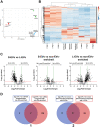
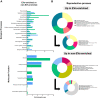
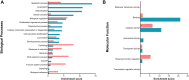
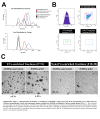
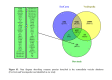
Seminal plasma contains many morphologically heterogeneous extracellular vesicles (sEVs). These are sequentially released by cells of the testis, epididymis, and accessory sex glands and involved in male and female reproductive processes. This study aimed to define in depth sEV subsets isolated by ultrafiltration and size exclusion chromatography, decode their proteomic profiles using liquid chromatography-tandem mass spectrometry, and quantify identified proteins using sequential window acquisition of all theoretical mass spectra. The sEV subsets were defined as large (L-EVs) or small (S-EVs) by their protein concentration, morphology, size distribution, and EV-specific protein markers and purity. Liquid chromatography-tandem mass spectrometry identified a total of 1034 proteins, 737 of them quantified by SWATH in S-EVs, L-EVs, and non-EVs-enriched samples (18-20 size exclusion chromatography-eluted fractions). The differential expression analysis revealed 197 differentially abundant proteins between both EV subsets, S-EVs and L-EVs, and 37 and 199 between S-EVs and L-EVs versus non-EVs-enriched samples, respectively. The gene ontology enrichment analysis of differentially abundant proteins suggested, based on the type of protein detected, that S-EVs could be mainly released through an apocrine blebbing pathway and be involved in modulating the immune environment of the female reproductive tract as well as during sperm-oocyte interaction. In contrast, L-EVs could be released by fusion of multivesicular bodies with the plasma membrane becoming involved in sperm physiological processes, such as capacitation and avoidance of oxidative stress. In conclusion, this study provides a procedure capable of isolating subsets of EVs from pig seminal plasma with a high degree of purity and shows differences in the proteomic profile between EV subsets, indicating different sources and biological functions for the sEVs.
Keywords: extracellular vesicles; pig; proteomics; seminal plasma; subsets.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no competing interests.
Figures


References
-
- Rodriguez-Martinez H., Kvist U., Ernerudh J., Sanz L., Calvete J.J. Seminal plasma proteins: what role do they play? Am. J. Reprod. Immunol. 2011;66:11–22. - PubMed
-
- Lin Y., Liang A., He Y., Li Z., Li Z., Wang G., et al. Proteomic analysis of seminal extracellular vesicle proteins involved in asthenozoospermia by iTRAQ. Mol. Reprod. Dev. 2019;86:1094–1105. - PubMed
-
- Leahy T., Rickard J.P., Pini T., Gadella B.M., de Graaf S.P. Quantitative proteomic analysis of seminal plasma, sperm membrane proteins, and seminal extracellular vesicles suggests vesicular mechanisms aid in the removal and addition of proteins to the ram sperm membrane. Proteomics. 2020;20 - PubMed

