Lung volume reduction surgery versus endobronchial valves: a randomised controlled trial
- PMID: 36796833
- PMCID: PMC10133584
- DOI: 10.1183/13993003.02063-2022
Lung volume reduction surgery versus endobronchial valves: a randomised controlled trial
Abstract
Background: Lung volume reduction surgery (LVRS) and bronchoscopic lung volume reduction (BLVR) with endobronchial valves can improve outcomes in appropriately selected patients with emphysema. However, no direct comparison data exist to inform clinical decision making in people who appear suitable for both procedures. Our aim was to investigate whether LVRS produces superior health outcomes when compared with BLVR at 12 months.
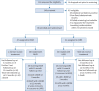
Methods: This multicentre, single-blind, parallel-group trial randomised patients from five UK hospitals, who were suitable for a targeted lung volume reduction procedure, to either LVRS or BLVR and compared outcomes at 1 year using the i-BODE score. This composite disease severity measure includes body mass index, airflow obstruction, dyspnoea and exercise capacity (incremental shuttle walk test). The researchers responsible for collecting outcomes were masked to treatment allocation. All outcomes were assessed in the intention-to-treat population.
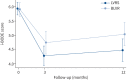
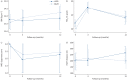
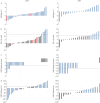
Results: 88 participants (48% female, mean±sd age 64.6±7.7 years, forced expiratory volume in 1 s percent predicted 31.0±7.9%) were recruited at five specialist centres across the UK and randomised to either LVRS (n=41) or BLVR (n=47). At 12 months follow-up, the complete i-BODE was available in 49 participants (21 LVRS/28 BLVR). Neither improvement in the i-BODE score (LVRS -1.10±1.44 versus BLVR -0.82±1.61; p=0.54) nor in its individual components differed between groups. Both treatments produced similar improvements in gas trapping (residual volume percent predicted: LVRS -36.1% (95% CI -54.6- -10%) versus BLVR -30.1% (95% CI -53.7- -9%); p=0.81). There was one death in each treatment arm.
Conclusion: Our findings do not support the hypothesis that LVRS is a substantially superior treatment to BLVR in individuals who are suitable for both treatments.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: D. Waller reports lecture honoraria from PulmonX Ltd, outside the submitted work. N.J. Greening reports grants from GSK, consulting fees from Genentech, lecture honoraria from AstraZeneca and Chiesi, travel support from Chiesi, outside the submitted work. P.L. Shah reports lecture honoraria from PulmonX Ltd, outside the submitted work. R. Bilancia reports lecture honoraria and travel support from Intuitive, outside the submitted work. R. Lawson is a Member of British Thoracic Society COPD Specialist Advisory group, a Member of South Yorkshire Clinical Senate, and a Member of South Yorkshire and Bassetlaw Respiratory Clinical Network, outside the submitted work. All other authors have nothing to disclose.
Figures


Comment in
-
Lung volume reduction in COPD: scope or surgery.Eur Respir J. 2023 Apr 27;61(4):2300353. doi: 10.1183/13993003.00353-2023. Print 2023 Apr. Eur Respir J. 2023. PMID: 37105590 No abstract available.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
