Effect of nintedanib in patients with systemic sclerosis-associated interstitial lung disease and risk factors for rapid progression
- PMID: 36796874
- PMCID: PMC9936273
- DOI: 10.1136/rmdopen-2022-002859
Effect of nintedanib in patients with systemic sclerosis-associated interstitial lung disease and risk factors for rapid progression
Abstract
Objective: To investigate the rate of decline in forced vital capacity (FVC), and the effect of nintedanib on the rate of decline in FVC, in subjects with systemic sclerosis-associated interstitial lung disease (SSc-ILD) who had risk factors for rapid decline in FVC.
Methods: The SENSCIS trial enrolled subjects with SSc and fibrotic ILD of ≥10% extent on high-resolution CT. The rate of decline in FVC over 52 weeks was analysed in all subjects and in those with early SSc (<18 months since first non-Raynaud symptom), elevated inflammatory markers (C reactive protein ≥6 mg/L and/or platelets ≥330×109/L) or significant skin fibrosis (modified Rodnan skin score (mRSS) 15-40 or mRSS ≥18) at baseline.
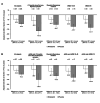
Results: In the placebo group, the rate of decline in FVC was numerically greater in subjects with <18 months since first non-Raynaud symptom (-167.8 mL/year), elevated inflammatory markers (-100.7 mL/year), mRSS 15-40 (-121.7 mL/year) or mRSS ≥18 (-131.7 mL/year) than in all subjects (-93.3 mL/year). Nintedanib reduced the rate of FVC decline across subgroups, with a numerically greater effect in patients with these risk factors for rapid FVC decline.
Conclusion: In the SENSCIS trial, subjects with SSc-ILD who had early SSc, elevated inflammatory markers or extensive skin fibrosis had a more rapid decline in FVC over 52 weeks than the overall trial population. Nintedanib had a numerically greater effect in patients with these risk factors for rapid ILD progression.
Keywords: Autoimmune Diseases; Pulmonary Fibrosis; Scleroderma, Systemic; Therapeutics.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DK reports grants from Bristol Myers Squibb, Horizon Therapeutics and Pfizer; consulting fees from AbbVie, BI, Bristol Myers Squibb, CSL Behring, Genentech, Horizon Therapeutics, Janssen, Prometheus, Talaris and Theraly; fees for presentations from AbbVie, BI, CSL Behring, Genentech, Horizon Therapeutics and Janssen; has a leadership or fiduciary role with Eicos; has received royalties or licences for the University of California Los Angeles Scleroderma Clinical Trials Consortium (SCTC) Gastrointestinal Tract instrument 2.0; and owns stock in Eicos. TMM reports consulting fees from AstraZeneca, Bayer, Blade Therapeutics, BI, Bristol Myers Squibb, Galapagos, Galecto, GlaxoSmithKline, IQVIA, Pliant, Respivant Sciences, Roche/Genentech, Theravance Biopharma and Veracyte; and fees for presentations from BI and Roche/Genentech. ERV reports grants from BI, Forbius, Kadmon and Horizon; and fees from BI for serving on advisory boards and for presentations. YA reports consulting fees from BI and Sanofi; fees for presentations from AbbVie, BI and Janssen; and has participated on Data Safety Monitoring Boards or advisory boards for BI, Chemomab, Curzion, Medsenic, Menarini, Prometheus and Sanofi. VS reports grants paid to her institution from the Belgian Fund for Scientific Research in Rheumatic Diseases, Research Foundation Flanders, BI and Janssen-Cilag; consulting fees and fees for presentations paid to herself and to her institution from BI; consulting fees paid to her institution from Janssen-Cilag; fees for presentations paid to her institution from Janssen-Cilag and UCB; support for travel paid to her institution by BI; and holds unpaid roles with the ACR and EULAR study groups on microcirculation, ERN-ReCONNET and the SCTC working group on capillaroscopy. SA reports grants paid to his institution from BI, Janssen and Momenta; consulting fees from AbbVie, AstraZeneca, BI, Corbus, CSL Behring and Novartis; and fees for presentations from Integrity Continuing Education. MKreuter reports grants, consulting fees and fees for presentations from BI and Roche; and holds leadership or fiduciary roles with Deutsche gesellschaft für Pneumologie, the European Respiratory Society and the German Respiratory Society. A-MH-V reports grants from BI; consulting fees from Arxx Therapeutics, Bayer, BI, Janssen, Lilly, Medscape, Merck Sharp & Dohme and Roche; fees for presentations from Arxx Therapeutics, Bayer, BI, Janssen, Lilly, Medscape, Merck Sharp & Dohme and Roche; support for travel from Actelion, BI, Medscape and Roche; and holds leadership or fiduciary roles with EUSTAR, the Nordic PH vision group and the Norwegian SSc study group. MKuwana reports grants paid to his institution from BI, MBL and Ono Pharmaceutical; consulting fees from BI, Chugai, Corbus and Mochida; fees for presentations from AbbVie, Asahi Kasei, Astellas, Bayer, BI, Chugai, Eisai, Janssen, Mitsubishi Tanabe and Ono Pharmaceutical; and has received royalties or licences from MBL. CS, MA and SS are employees of BI. CPD reports grants from Arxx Therapeutics, CSL Behring, GlaxoSmithKline, Inventiva and Servier; consulting fees from AbbVie, Acceleron, Bayer, BI, Corbus, CSL Behring, GlaxoSmithKline, Horizon Therapeutics, Inventiva, Roche and Sanofi; and fees for presentations from BI, Corbus and Janssen.
Figures
Similar articles
-
Nintedanib in Patients With Systemic Sclerosis-Associated Interstitial Lung Disease: Subgroup Analyses by Autoantibody Status and Modified Rodnan Skin Thickness Score.Arthritis Rheumatol. 2022 Mar;74(3):518-526. doi: 10.1002/art.41965. Epub 2022 Feb 13. Arthritis Rheumatol. 2022. PMID: 34514739 Free PMC article. Clinical Trial.
-
Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial.Lancet Respir Med. 2021 Jan;9(1):96-106. doi: 10.1016/S2213-2600(20)30330-1. Lancet Respir Med. 2021. PMID: 33412120 Clinical Trial.
-
Decline in forced vital capacity in subjects with systemic sclerosis-associated interstitial lung disease in the SENSCIS trial compared with healthy reference subjects.Respir Res. 2022 Jul 5;23(1):178. doi: 10.1186/s12931-022-02095-6. Respir Res. 2022. PMID: 35790961 Free PMC article.
-
Interstitial lung disease in patients with systemic sclerosis: what can we learn from the SENSCIS trial?Clin Exp Rheumatol. 2023 Aug;41(8):1713-1719. doi: 10.55563/clinexprheumatol/trcv91. Epub 2023 Aug 3. Clin Exp Rheumatol. 2023. PMID: 37534955 Review.
-
Nintedanib Therapy Alone and Combined with Mycophenolate in Patients with Systemic Sclerosis-associated Interstitial Lung Disease: Systematic Reviews and Meta-analysis.Ann Am Thorac Soc. 2024 Mar;21(3):474-485. doi: 10.1513/AnnalsATS.202301-081OC. Ann Am Thorac Soc. 2024. PMID: 37773000 Free PMC article.
Cited by
-
Outcomes of systemic sclerosis associated interstitial lung disease patients with a persistent inflammatory phenotype based on serial CRP measurements.Thorax. 2023 Dec;78(12):1166-1167. doi: 10.1136/thorax-2023-220820. Epub 2023 Oct 5. Thorax. 2023. PMID: 37798113 Free PMC article. No abstract available.
-
Development of a multivariable prediction model for progression of systemic sclerosis-associated interstitial lung disease.RMD Open. 2024 Sep 5;10(3):e004240. doi: 10.1136/rmdopen-2024-004240. RMD Open. 2024. PMID: 39242112 Free PMC article.
-
Towards a Better Prognosis in Patients with Systemic Sclerosis-Related Pulmonary Arterial Hypertension: Recent Developments and Perspectives.J Clin Med. 2024 Sep 30;13(19):5834. doi: 10.3390/jcm13195834. J Clin Med. 2024. PMID: 39407897 Free PMC article. Review.
-
Mesenchymal stem cell-derived extracellular vesicles in systemic sclerosis: role and therapeutic directions.Front Cell Dev Biol. 2024 Oct 17;12:1492821. doi: 10.3389/fcell.2024.1492821. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39483335 Free PMC article. Review.
-
Heterogeneity of determining disease severity, clinical course and outcomes in systemic sclerosis-associated interstitial lung disease: a systematic literature review.RMD Open. 2023 Nov;9(4):e003426. doi: 10.1136/rmdopen-2023-003426. RMD Open. 2023. PMID: 37940340 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
