Regulatory implications of ctDNA in immuno-oncology for solid tumors
- PMID: 36796877
- PMCID: PMC9936292
- DOI: 10.1136/jitc-2022-005344
Regulatory implications of ctDNA in immuno-oncology for solid tumors
Abstract
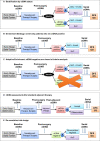
In the era of precision oncology, use of circulating tumor DNA (ctDNA) is emerging as a minimally invasive approach for the diagnosis and management of patients with cancer and as an enrichment tool in clinical trials. In recent years, the US Food and Drug Administration has approved multiple ctDNA-based companion diagnostic assays for the safe and effective use of targeted therapies and ctDNA-based assays are also being developed for use with immuno-oncology-based therapies. For early-stage solid tumor cancers, ctDNA may be particularly important to detect molecular residual disease (MRD) to support early implementation of adjuvant or escalated therapy to prevent development of metastatic disease. Clinical trials are also increasingly using ctDNA MRD for patient selection and stratification, with an ultimate goal of improving trial efficiency through use of an enriched patient population. Standardization and harmonization of ctDNA assays and methodologies, along with further clinical validation of ctDNA as a prognostic and predictive biomarker, are necessary before ctDNA may be considered as an efficacy-response biomarker to support regulatory decision making.
Keywords: Clinical Trials as Topic; Immunotherapy; Review.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- FDA draft guidance for industry: use of circulating tumor DNA for early stage solid tumor drug development. Available: https://www.fda.gov/media/158072/download [Accessed 31 May 2022].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
