Study protocol for a randomised controlled trial of a virtual antenatal intervention for improved diet and iron intake in Kapilvastu district, Nepal: VALID
- PMID: 36797013
- PMCID: PMC9936277
- DOI: 10.1136/bmjopen-2022-064709
Study protocol for a randomised controlled trial of a virtual antenatal intervention for improved diet and iron intake in Kapilvastu district, Nepal: VALID
Abstract
Introduction: Despite evidence that iron and folic acid (IFA) supplements can improve anaemia in pregnant women, uptake in Nepal is suboptimal. We hypothesised that providing virtual counselling twice in mid-pregnancy, would increase compliance to IFA tablets during the COVID-19 pandemic compared with antenatal care (ANC alone.
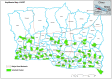
Methods and analysis: This non-blinded individually randomised controlled trial in the plains of Nepal has two study arms: (1) control: routine ANC; and (2) 'Virtual' antenatal counselling plus routine ANC. Pregnant women are eligible to enrol if they are married, aged 13-49 years, able to respond to questions, 12-28 weeks' gestation, and plan to reside in Nepal for the next 5 weeks. The intervention comprises two virtual counselling sessions facilitated by auxiliary nurse midwives at least 2 weeks apart in mid-pregnancy. Virtual counselling uses a dialogical problem-solving approach with pregnant women and their families. We randomised 150 pregnant women to each arm, stratifying by primigravida/multigravida and IFA consumption at baseline, providing 80% power to detect a 15% absolute difference in primary outcome assuming 67% prevalence in control arm and 10% loss-to-follow-up. Outcomes are measured 49-70 days after enrolment, or up to delivery otherwise.
Primary outcome: consumption of IFA on at least 80% of the previous 14 days.
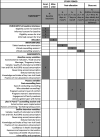
Secondary outcomes: dietary diversity, consumption of intervention-promoted foods, practicing ways to enhance bioavailability and knowledge of iron-rich foods. Our mixed-methods process evaluation explores acceptability, fidelity, feasibility, coverage (equity and reach), sustainability and pathways to impact. We estimate costs and cost-effectiveness of the intervention from a provider perspective. Primary analysis is by intention-to-treat, using logistic regression.
Ethics and dissemination: We obtained ethical approval from Nepal Health Research Council (570/2021) and UCL ethics committee (14301/001). We will disseminate findings in peer-reviewed journal articles and by engaging policymakers in Nepal.
Trial registration number: ISRCTN17842200.
Keywords: epidemiology; nutrition & dietetics; public health.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
