1'-O-methyl-averantin isolated from the endolichenic fungus Jackrogersella sp. EL001672 suppresses colorectal cancer stemness via sonic Hedgehog and Notch signaling
- PMID: 36797277
- PMCID: PMC9935543
- DOI: 10.1038/s41598-023-28773-z
1'-O-methyl-averantin isolated from the endolichenic fungus Jackrogersella sp. EL001672 suppresses colorectal cancer stemness via sonic Hedgehog and Notch signaling
Abstract
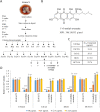
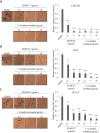
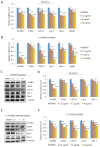
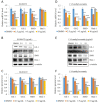
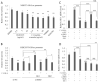
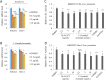
Endolichenic fungi are host organisms that live on lichens and produce a wide variety of secondary metabolites. Colorectal cancer stem cells are capable of self-renewal and differentiation into cancer cells, which makes cancers difficult to eradicate. New alternative therapeutics are needed to inhibit the growth of tumor stem cells. This study examined the ability of an extract of Jackrogersella sp. EL001672 (derived from the lichen Cetraria sp.) and the isolated compound 1'-O-methyl-averantin to inhibit development of cancer stemness. The endolichenic fungus Jackrogersella sp. EL001672 (KACC 83021BP), derived from Cetraria sp., was grown in culture medium. The culture broth was extracted with acetone to obtain a crude extract. Column chromatography and reverse-phase HPLC were used to isolate an active compound. The anticancer activity of the extract and the isolated compound was evaluated by qRT-PCR and western blotting, and in cell viability, spheroid formation, and reporter assays. The acetone extract of EL001672 did not affect cell viability. However, 1'-O-methyl-averantin showed cytotoxic effects against cancer cell lines at 50 μg/mL and 25 μg/mL. Both the crude extract and 1'-O-methyl-averantin suppressed spheroid formation in CRC cell lines, and downregulated expression of stemness markers ALDH1, CD44, CD133, Lgr-5, Msi-1, and EphB1. To further characterize the mechanism underlying anti-stemness activity, we examined sonic Hedgehog and Notch signaling. The results showed that the crude extract and the 1'-O-methyl-averantin inhibited Gli1, Gli2, SMO, Bmi-1, Notch-1, Hes-1, and the CSL complex. Consequently, an acetone extract and 1'-O-methyl-averantin isolated from EL001672 suppresses colorectal cancer stemness by regulating the sonic Hedgehog and Notch signaling pathways.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

