A trans-kingdom T6SS effector induces the fragmentation of the mitochondrial network and activates innate immune receptor NLRX1 to promote infection
- PMID: 36797302
- PMCID: PMC9935632
- DOI: 10.1038/s41467-023-36629-3
A trans-kingdom T6SS effector induces the fragmentation of the mitochondrial network and activates innate immune receptor NLRX1 to promote infection
Abstract
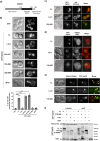
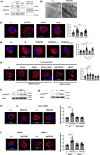
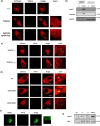
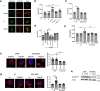
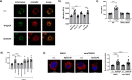
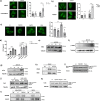
Bacteria can inhibit the growth of other bacteria by injecting effectors using a type VI secretion system (T6SS). T6SS effectors can also be injected into eukaryotic cells to facilitate bacterial survival, often by targeting the cytoskeleton. Here, we show that the trans-kingdom antimicrobial T6SS effector VgrG4 from Klebsiella pneumoniae triggers the fragmentation of the mitochondrial network. VgrG4 colocalizes with the endoplasmic reticulum (ER) protein mitofusin 2. VgrG4 induces the transfer of Ca2+ from the ER to the mitochondria, activating Drp1 (a regulator of mitochondrial fission) thus leading to mitochondrial network fragmentation. Ca2+ elevation also induces the activation of the innate immunity receptor NLRX1 to produce reactive oxygen species (ROS). NLRX1-induced ROS limits NF-κB activation by modulating the degradation of the NF-κB inhibitor IκBα. The degradation of IκBα is triggered by the ubiquitin ligase SCFβ-TrCP, which requires the modification of the cullin-1 subunit by NEDD8. VgrG4 abrogates the NEDDylation of cullin-1 by inactivation of Ubc12, the NEDD8-conjugating enzyme. Our work provides an example of T6SS manipulation of eukaryotic cells via alteration of the mitochondria.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

