The deficiency of poly-β-1,6-N-acetyl-glucosamine deacetylase trigger A. baumannii to convert to biofilm-independent colistin-tolerant cells
- PMID: 36797306
- PMCID: PMC9935895
- DOI: 10.1038/s41598-023-30065-5
The deficiency of poly-β-1,6-N-acetyl-glucosamine deacetylase trigger A. baumannii to convert to biofilm-independent colistin-tolerant cells
Abstract
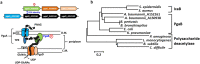
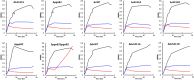
Acinetobacter baumannii is a nosocomial pathogen that can be resistant to antibiotics by rapidly modulating its anti-drug mechanisms. The multidrug-resistant A. baumannii has been considered one of the most threatening pathogens to our society. Biofilm formation and persistent cells within the biofilm matrix are recognized as intractable problems, especially in hospital-acquired infections. Poly-β-1,6-N-acetyl-glucosamine (PNAG) is one of the important building blocks in A. baumannii's biofilm. Here, we discover a protein phosphoryl-regulation on PNAG deacetylase, AbPgaB1, in which residue Ser411 was phosphorylated. The phosphoryl-regulation on AbPgaB1 modulates the product turnover rate in which deacetylated PNAG is produced and reflected in biofilm production. We further uncovered the PgaB deficient A. baumannii strain shows the lowest level of biofilm production but has a high minimal inhibition concentration to antibiotic colistin and tetracycline. Based on bactericidal post-antibiotic effects and time-dependent killing assays with antibacterial drugs, we claim that the PgaB-deficient A. baumannii converts to colistin-tolerant cells. This study utilizes a biofilm-independent colistin-tolerant model of A. baumannii to further investigate its characteristics and mechanisms to better understand clinical outcomes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Effects of Sub-Minimum Inhibitory Concentrations of Imipenem and Colistin on Expression of Biofilm-Specific Antibiotic Resistance and Virulence Genes in Acinetobacter baumannii Sequence Type 1894.Int J Mol Sci. 2022 Oct 21;23(20):12705. doi: 10.3390/ijms232012705. Int J Mol Sci. 2022. PMID: 36293559 Free PMC article.
-
Biofilm Formation and Colistin Susceptibility of Acinetobacter baumannii Isolated from Korean Nosocomial Samples.Microb Drug Resist. 2015 Aug;21(4):452-7. doi: 10.1089/mdr.2014.0236. Epub 2015 Feb 25. Microb Drug Resist. 2015. PMID: 25714496
-
Highly synergistic activity of melittin with imipenem and colistin in biofilm inhibition against multidrug-resistant strong biofilm producer strains of Acinetobacter baumannii.Eur J Clin Microbiol Infect Dis. 2018 Mar;37(3):443-454. doi: 10.1007/s10096-018-3189-7. Epub 2018 Jan 20. Eur J Clin Microbiol Infect Dis. 2018. PMID: 29353377
-
[Influence of poly-β-1-6-N-acetylglucosamine on biofilm formation and drug resistance of Acinetobacter baumannii].Zhonghua Shao Shang Za Zhi. 2015 Feb;31(1):45-7. Zhonghua Shao Shang Za Zhi. 2015. PMID: 25876639 Review. Chinese.
-
Factors mediating Acinetobacter baumannii biofilm formation: Opportunities for developing therapeutics.Curr Res Microb Sci. 2022 Mar 28;3:100131. doi: 10.1016/j.crmicr.2022.100131. eCollection 2022. Curr Res Microb Sci. 2022. PMID: 35909621 Free PMC article. Review.
Cited by
-
The activity of hydrolytic enzymes and antibiotics against biofilms of bacteria isolated from industrial-scale cooling towers.Microb Cell Fact. 2024 Oct 16;23(1):282. doi: 10.1186/s12934-024-02502-1. Microb Cell Fact. 2024. PMID: 39415191 Free PMC article.
References
-
- del Mar Tomás M, et al. Cloning and functional analysis of the gene encoding the 33- to 36- kilodalton outer membrane protein associated with carbapenem resistance in Acinetobacter baumannii. Antimicrob. Agents. Chemother. 2005;49:5172–5175. doi: 10.1128/AAC.49.12.5172-5175.2005. - DOI - PMC - PubMed
-
- Fernández-Cuenca F, et al. Relationship between beta-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 2003;51:565–574. doi: 10.1093/jac/dkg097. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

