Bidirectional alterations in brain temperature profoundly modulate spatiotemporal neurovascular responses in-vivo
- PMID: 36797344
- PMCID: PMC9935519
- DOI: 10.1038/s42003-023-04542-6
Bidirectional alterations in brain temperature profoundly modulate spatiotemporal neurovascular responses in-vivo
Abstract
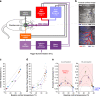
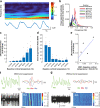
Neurovascular coupling (NVC) is a mechanism that, amongst other known and latent critical functions, ensures activated brain regions are adequately supplied with oxygen and glucose. This biological phenomenon underpins non-invasive perfusion-related neuroimaging techniques and recent reports have implicated NVC impairment in several neurodegenerative disorders. Yet, much remains unknown regarding NVC in health and disease, and only recently has there been burgeoning recognition of a close interplay with brain thermodynamics. Accordingly, we developed a novel multi-modal approach to systematically modulate cortical temperature and interrogate the spatiotemporal dynamics of sensory-evoked NVC. We show that changes in cortical temperature profoundly and intricately modulate NVC, with low temperatures associated with diminished oxygen delivery, and high temperatures inducing a distinct vascular oscillation. These observations provide novel insights into the relationship between NVC and brain thermodynamics, with important implications for brain-temperature related therapies, functional biomarkers of elevated brain temperature, and in-vivo methods to study neurovascular coupling.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

