Serological response to vaccination in post-acute sequelae of COVID
- PMID: 36797666
- PMCID: PMC9933819
- DOI: 10.1186/s12879-023-08060-y
Serological response to vaccination in post-acute sequelae of COVID
Abstract
Background: Individuals with post-acute sequelae of COVID (PASC) may have a persistence in immune activation that differentiates them from individuals who have recovered from COVID without clinical sequelae. To investigate how humoral immune activation may vary in this regard, we compared patterns of vaccine-provoked serological response in patients with PASC compared to individuals recovered from prior COVID without PASC.
Methods: We prospectively studied 245 adults clinically diagnosed with PASC and 86 adults successfully recovered from prior COVID. All participants had measures of humoral immunity to SARS-CoV-2 assayed before or after receiving their first-ever administration of COVID vaccination (either single-dose or two-dose regimen), including anti-spike (IgG-S and IgM-S) and anti-nucleocapsid (IgG-N) antibodies as well as IgG-S angiotensin-converting enzyme 2 (ACE2) binding levels. We used unadjusted and multivariable-adjusted regression analyses to examine the association of PASC compared to COVID-recovered status with post-vaccination measures of humoral immunity.
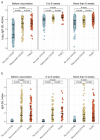
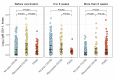
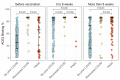
Results: Individuals with PASC mounted consistently higher post-vaccination IgG-S antibody levels when compared to COVID-recovered (median log IgG-S 3.98 versus 3.74, P < 0.001), with similar results seen for ACE2 binding levels (median 99.1 versus 98.2, P = 0.044). The post-vaccination IgM-S response in PASC was attenuated but persistently unchanged over time (P = 0.33), compared to in COVID recovery wherein the IgM-S response expectedly decreased over time (P = 0.002). Findings remained consistent when accounting for demographic and clinical variables including indices of index infection severity and comorbidity burden.
Conclusion: We found evidence of aberrant immune response distinguishing PASC from recovered COVID. This aberrancy is marked by excess IgG-S activation and ACE2 binding along with findings consistent with a delayed or dysfunctional immunoglobulin class switching, all of which is unmasked by vaccine provocation. These results suggest that measures of aberrant immune response may offer promise as tools for diagnosing and distinguishing PASC from non-PASC phenotypes, in addition to serving as potential targets for intervention.
Keywords: Anti-spike antibody; Immune activation; Post-acute sequelae; SARS-CoV-2; Serological response.
© 2023. The Author(s).
Conflict of interest statement
JCP, ECF, and JLS work for Abbott Diagnostics, a company that performed the serological assays on the biospecimens that were collected for this study. The remaining authors have no potential conflicts of interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

