Recent advances of small extracellular vesicle biomarkers in breast cancer diagnosis and prognosis
- PMID: 36797736
- PMCID: PMC9933347
- DOI: 10.1186/s12943-023-01741-x
Recent advances of small extracellular vesicle biomarkers in breast cancer diagnosis and prognosis
Abstract
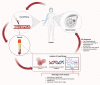
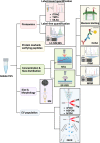
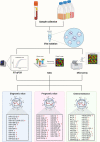
Current clinical tools for breast cancer (BC) diagnosis are insufficient but liquid biopsy of different bodily fluids has recently emerged as a minimally invasive strategy that provides a real-time snapshot of tumour biomarkers for early diagnosis, active surveillance of progression, and post-treatment recurrence. Extracellular vesicles (EVs) are nano-sized membranous structures 50-1000 nm in diameter that are released by cells into biological fluids. EVs contain proteins, nucleic acids, and lipids which play pivotal roles in tumourigenesis and metastasis through cell-to-cell communication. Proteins and miRNAs from small EVs (sEV), which range in size from 50-150 nm, are being investigated as a potential source for novel BC biomarkers using mass spectrometry-based proteomics and next-generation sequencing. This review covers recent developments in sEV isolation and single sEV analysis technologies and summarises the sEV protein and miRNA biomarkers identified for BC diagnosis, prognosis, and chemoresistance. The limitations of current sEV biomarker research are discussed along with future perspective applications.
Keywords: Biomarker; Breast cancer; Diagnosis; Extracellular vesicles; Liquid biopsy; Prognosis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Group USCSW. U.S. Cancer Statistics Data Visualizations Tool, Based on 2019 Submission Data (1999–2017). US Department of Health and Human Services. 2020.
-
- Indicators CAsNCC. Relative Survival by Stage at Diagnosis (Female Breast Cancer) 2019. https://ncci.canceraustralia.gov.au/relative-survival-stage-diagnosis-fe.... Accessed 20 Sept 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

