Recent advances and applications of CRISPR-Cas9 in cancer immunotherapy
- PMID: 36797756
- PMCID: PMC9933290
- DOI: 10.1186/s12943-023-01738-6
Recent advances and applications of CRISPR-Cas9 in cancer immunotherapy
Abstract
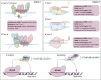
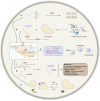
The incidence and mortality of cancer are the major health issue worldwide. Apart from the treatments developed to date, the unsatisfactory therapeutic effects of cancers have not been addressed by broadening the toolbox. The advent of immunotherapy has ushered in a new era in the treatments of solid tumors, but remains limited and requires breaking adverse effects. Meanwhile, the development of advanced technologies can be further boosted by gene analysis and manipulation at the molecular level. The advent of cutting-edge genome editing technology, especially clustered regularly interspaced short palindromic repeats (CRISPR-Cas9), has demonstrated its potential to break the limits of immunotherapy in cancers. In this review, the mechanism of CRISPR-Cas9-mediated genome editing and a powerful CRISPR toolbox are introduced. Furthermore, we focus on reviewing the impact of CRISPR-induced double-strand breaks (DSBs) on cancer immunotherapy (knockout or knockin). Finally, we discuss the CRISPR-Cas9-based genome-wide screening for target identification, emphasis the potential of spatial CRISPR genomics, and present the comprehensive application and challenges in basic research, translational medicine and clinics of CRISPR-Cas9.
Keywords: CRISPR-Cas9; Cancer immunotherapy; Cellular therapy; Mechanism; NK/macrophage.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

