The therapeutic effect of larval saliva and hemolymph of Lucilia sericata on the treatment of Leishmania major lesion in BALB/c mice946
- PMID: 36797798
- PMCID: PMC9936726
- DOI: 10.1186/s13071-023-05660-0
The therapeutic effect of larval saliva and hemolymph of Lucilia sericata on the treatment of Leishmania major lesion in BALB/c mice946
Abstract
Background: Treatment of cutaneous leishmaniasis (CL) remains a major challenge for the public health and medical community. It has been claimed that natural compounds derived from fly larvae have anti-leishmania properties against some species of Leishmania. The present study aimed at assessing the in vitro effects of larval products of Lucilia sericata against the promastigote and intracellular amastigote forms of Leishmania major. Also, the therapeutic effect of larval products on lesions induced by L. major infection was evaluated in BALB/c mice models.
Methods: Parasite specimens and macrophage cells were exposed to varying concentrations of larval products for 24-120 h. Lesion progression and parasite load were investigated in the models to assess the therapeutic effects of the products.
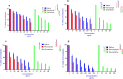
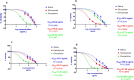
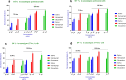
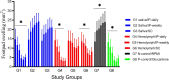
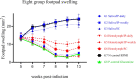
Results: The larval products displayed more potent cytotoxicity against L. major promastigotes. The IC50 values for larval saliva and hemolymph were 100.6 and 37.96 ug/ml, respectively. The IC50 of glucantime was 9.480 ug/ml. Also, the saliva and hemolymph of L. sericata exhibited higher cytotoxicity against the promastigotes of L. major but were less toxic to the macrophage cells. Treatment with leishmanicidal agents derived from larvae of L. sericata decreased the infection rate and the number of amastigotes per infected host cell at all concentrations. Lesion size was significantly (F (7, 38) = 8.54, P < 0.0001) smaller in the treated mice compared with the untreated control group. The average parasite burden in the treated mice groups (1.81 ± 0.74, 1.03 ± 0.45 and 3.37 ± 0.41) was similar to the group treated with a daily injection of glucantime (1.77 ± 0.99) and significantly lower (F (7, 16) = 66.39, P < 0.0001) than in the untreated control group (6.72 ± 2.37).
Conclusions: The results suggest that the larval products of L. sericata were effective against L. major parasites both in vivo and in vitro. However, more clinical trial studies are recommended to evaluate the effects of these larval products on human subjects.
Keywords: Cutaneous leishmaniasis; Glucantime; Leishmania major; Leishmanial activity; Lucilia sericata; Natural compound.
© 2023. The Author(s).
Conflict of interest statement
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Figures





References
-
- Centers for Disease Control and Prevention 2020 [Internet]. [cited 2020 Feb 18]. https://www.cdc.gov/parasites/.
-
- World Health Organization 2021 [Internet]. [cited 2021 May 20]. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
-
- Kassebaum NJ, Arora M, Barber RM, Bhutta ZA, Brown J, Carter A, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global burden of disease study 2015. Lancet. 2016;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

